Sandbox Reserved 779
From Proteopedia
[[Image:Example.jpg]
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
β-Lactoglobulin
|
Contents |
β-Lactoglobulin
β-Lactoglobulin (β-LG) was first isolated in 1934.[1] It is the main globular protein of whey, a by-product from cow milk-cheese manufacture. Cow milk itself contains 20% whey proteins and 80% casein protein. β-Lactoglobulin constitutes 50-65% dry solids whey protein or 12% of whole cow milk proteins. Due to its abundance, and relatively easy to isolate nature, β-Lactoglobulin used widely in Industry to increase the protein contents of the food and beverage products. Bovine β-lactoglobulin (β-Lg) is a commercially important whey protein with undetermined biological function, although it is of obvious nutritional value. β-Lg binds a variety of ligands, and it appears that there are at least 3 independent binding sites: calyx, putative grove, and dimer interface (Fig. 2).[2][3] β-Lactoglobulin amino-acid sequence and 3-dimensional structure show that it belongs to Lipocalin family which capable of binding hydrophobic ligands and thus may act as specific transporters, as does serum retinol binding protein. [4] Bovine β-Lactoglobulin is synthesized in cow mammary gland and secreted in the milk. It causes an allergic reaction in human and is one of the causes of cow's milk allergy.
Lipocalin Proteins
β-Lactoglobulin belongs to the calycin superfamily and Lipocalin family. Lipocalins are typically small (160-180 residues in length), extracellular proteins and able to bind small hydrophobic molecules (such as retinol); bind to specific cell-surface receptors; and form of covalent and non-covalent complexes with other soluble macromolecules. Lipocalin proteins have also been classified mainly as transport proteins. [5]
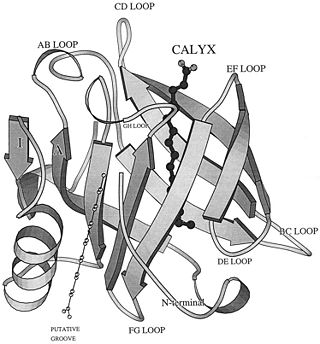
The lipocalin family is a large and diverse family of proteins with functions varying from insect camouflage to small hydrophobic molecule transport typified by the serum retinol-binding protein [6] The crystal structures so far determined reveal the typical lipocalin to be an eight-stranded antiparallel β-barrel arranged to form a conical central calyx or cavity in which the hydrophobic ligand is located.[7]
Structure of β-Lactoglobulin
β-Lactoglobulin is a small globulin protein, soluble in dilute salt solution with 162 amino acid residues (Mr ∼18,400 Daltons) for each monomer that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand.
Residues and secondary structures
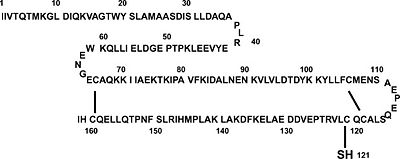
β-Lactoglobulin contains two disulfide bonds (Cys 66–Cys 160 and Cys 106–Cys 119) and a free thiol (Cys 121)(Fig. 1). Structures of βLG have been reported by several groups with X-ray crystallography and solution NMR, that it is predominantly β-sheet protein. The β-barrel, or so called calyx, is conical and is made of two β-sheets: the B–D strands and N-terminal half of the A strand (denoted AN) form one sheet, and the E–H strands and C-terminal half of the A strand (denoted AC) form the other. On the outer surface of the β-barrel, between the G and H strands, is the 3-turn α-helix. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG, are generally quite short, whereas those at the open end, AB, CD, EF,and GH, are significantly longer and more flexible. In the calyx, there is a large central cavity which is surrounded by hydrophobic residues and is accessible to solvent. This cavity provides the principal ligand-binding site (Fig. 2). [10]
Genetic Variants
Genetically, β-lactoglobulin may exist as one of several variants, among which the variants A and B are the most abundant. The A and B variants of the protein differ from each other by amino acid residues at positions Asp64 (Gly64 in variant B) and Val118 (Ala118 in variant B). These differences in primary structure render the two variants slightly different with respect to isoelectric point, solubility, self-association properties, as well as pressure and temperature stability. However, the structural characteristics of the A and B variants of bovine b-lactoglobulin are virtually indistinguishable. In its native state, β-lactoglobulin is a predominantly β-sheet protein containing nine b-strands and three a-helices. The core of the protein is formed by a flattened b-barrel (a calyx) composed of eight antiparallel b-strands (A to H).[11]

Dimer/Monomer
At physiological conditions, majority of bovine b-lactoglobulin forms a dimer (Fig. 3). Below pH 3, the dimer dissociates into monomers which maintain their native conformation. Dimeric Lactoglobulin molecules exist in the open conformation at basic pH, whereas they exist in the closed conformation at acidic pH, after undergoing Tanford transition around neutral pH.[12]
Ligands and Active sites
The true function of β-Lg is unknown, but it has been suggested that it is involved in the transport of retinol and/or fatty acids. It binds
