From Proteopedia
proteopedia linkproteopedia link
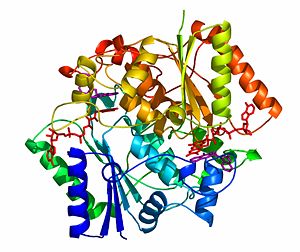
The crystal structure of NADH quinone oxidoreductase (NQO1) in complex with its potent inhibitor dicoumarol
NAD(P)H quinone oxidoreductase 1 (NQO1, EC 1.6.5.2) is a ubiquitous flavoenzyme that catalyzes two electron reduction of quinones to hydroquinones utilizing NAD(P)H as an electron donor.
NQO1 is a homo-dimer that functions via a “ping pong” mechanism. NAD(P)H binds to NQO1, reduces the FAD co-factor and is then released, allowing the quinone substrate to bind the enzyme and to be reduced. The NAD(P)H and the quinone binding sites of NQO1 have a significant overlap, thus providing a molecular basis for this “ping pong” mechanism.
Certain
coumarins,
flavones and the reactive dye cibacron blue are
competitive inhibitors of NQO1 activity, which compete with NAD(P)H for binding to NQO1.
Dicoumarol (3-3’–methylene-bis (4-hydroxycoumarin)),
is the most potent competitive inhibitor of NQO1. Dicoumarol competes with NAD(P)H for binding to NQO1 and prevents the
electron transfer to FAD.
In addition to its role in the detoxification of quinones, NQO1 is also a 20S proteasome-associated protein that plays an important role in the stability of the tumor suppressor p53 and several other short-lived proteins including p73α and ornithine decarboxylase (ODC, i.e. 7odc). NQO1 binds and stabilizes p53, protecting p53 from ubiquitin-independent 20S proteasomal degradation. Dicoumarol and several other inhibitors of NQO1 activity, which compete with NADH for binding to NQO1, disrupt the binding of NQO1 to p53 and induce ubiquitin-independent p53 degradation.
|
The crystal structure of human NQO1 in complex with dicoumarol was determined at 2.75 Å resolution (2f1o). NQO1 is a composed of two interlocked monomers. are formed and are present at the dimer interface (FAD is colored red and dicoumarol is colored blue). Therefore, each from these two is formed by both monomers. Dicoumarol is colored cyan, FAD in orange, nitrogens and oxygens are shown in CPK colors. NQO1 chain A is colored blueviolet and chain C in lime. NQO1 residues, participating in ligand interactions, are shown as stick representation and are labeled (A and C refer to the NQO1 chains). H-bonds are shown by dashed lines with their distances.
of the active site of dicoumarol/hNQO1 complex (residues important for ligand interactions are colored magenta) with that of apo hNQO1 dimer (1d4a, residues important for ligand interactions are colored blue) reveals that structural changes associated with dicoumarol binding occur on several residues involving both monomers. Dicoumarol is colored in cyan; FAD is colored in orange. The RMSD between the apo hNQO1 (1d4a) and hNQO1 in complex with dicoumarol is 0.36Å for the 546 Cα atoms. The dicoumarol-hNQO1 binding causes several structural changes. The most prominent of them is Tyr 128 and Phe 232 movement in the first monomer. These residues are located on the surface of the NQO1 catalytic pocket. The between these residues increases from ~5 Å in the apo hNQO1 to ~12 Å in the dicoumarol/hNQO1 complex.
Quinones (including duroquinone (2,3,5,6-tetramethyl-p-benzoquinone) are substrates of NQO1 (it catalyzes two-electron reduction of them to hydroquinones). Duroquinone (yellow) binds to the by interactions involving the FAD and several hydrophobic and hydrophilic residues in the duroquinone-NQO1 complex (1dxo). The structure of the hNQO1 dimer in complex with duroquinone is also similar to that of hNQO1 in complex with dicoumarol (RMSD is 0.33Å for the 546 Cα atoms). In this case, the main differences between these two structures, as well as to that of apo hNQO1, involve the distance between residues of the first monomer. The FAD molecule has very similar conformation in both hNQO1-duroquinone (pink) and hNQO1−dicoumarol (orange) complexes. Based on the comparison of NQO1 structure in complex with different NQO1 inhibitors and our previous analysis of NQO1 mutations that affect NQO1 interactions we propose that the specific conformation of Tyr 128 and Phe 232 is important for NQO1 interaction with p53 and other client proteins.
The quinone ES936 causes irreversible inhibition of the NQO1. of the hNQO1 dimer in complex with ES936 (red) (1kbq) with the hNQO1−dicoumarol complex (2f1o) yields 0.45Å RMSD for the 546 Cα atoms. The ES936 causes structural change only in the position of Phe 232. The movement of this residue is smaller than that caused by dicoumarol. The between Tyr 128 and Phe 232 in the hNQO1−ES936 complex is only ~7 Å, while in the hNQO1−dicoumarol complex it is ~12 Å.
|
3D structures of NQ01
Quinone reductase
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3177-82. PMID:10706635 doi:http://dx.doi.org/10.1073/pnas.050585797
- Asher G, Dym O, Tsvetkov P, Adler J, Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006 May 23;45(20):6372-8. PMID:16700548 doi:10.1021/bi0600087