FERRIC ENTEROBACTIN RECEPTOR (1fep)
FepA is an outer membrane protein which functions to transport ferric enterobactin into the periplasm.
Since the outer membrane lacks local access to established ion gradient or ATP, the proton motive force is harnessed as the energy source by a complex of cytoplasmic membrane proteins - TonB, ExbB, and ExbD.
Similar to the other outer membrane proteins, FepA serves as a receptor for colicins B and D [2].
Structure
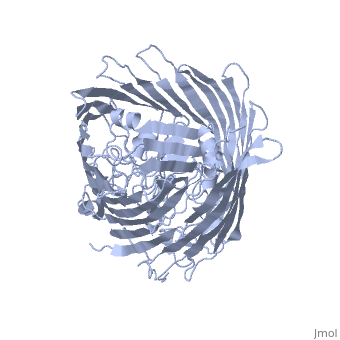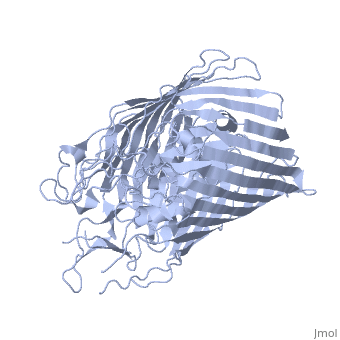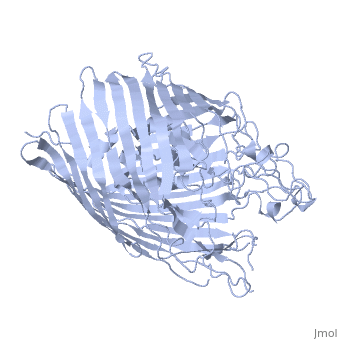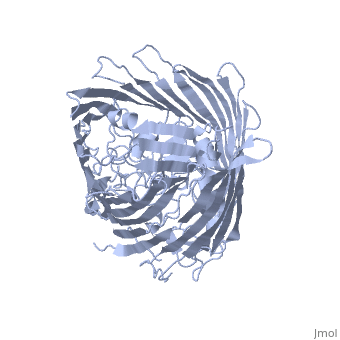
FepA is a 724-residue protein composed of two domains: a 22-stranded antiparallel and an N-terminal folded into the barrel pore (residues 1-153). The molecule is approximately 70 Å high with a cross-section of 40 Å • 30Å. The strands are connected via long ; several of the loops extend 30-40 Å and may facilitate the initial binding of ferric enterobactin[3]
It has been shown that mutations in the globular domain prevent transport of ligands and interaction with TonB. Deletions in the globular domain of FepA also impair in vivo cross-linking to TonB[4] (which has been earlier demonstrated to be greatly enhanced by the presence of ligand[5]). Mutations in the (residues 12-18) can abolish the interaction with TonB; however, the recognition region of FepA may involve more than the TonB box alone[3].
Sequence comparisons also showed the significance of the N-terminal domain: the homology regions are conserved among FepA, ferrichrome receptor, vitamin B12 receptor, and aerobactin receptor. Highly include Arg 75, Gly 76, Gly 127, Gly 134, Arg 431, Glu 511, Gly 565, Asn 677 and Arg 714 [3]