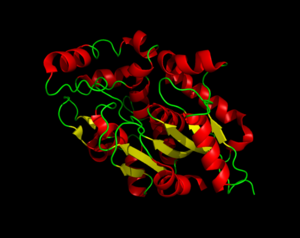
Three dimensional structure of Palmitoyl-Protein Thioesterase 1.
Palmitoyl-Protein Thioesterase 1 (PPT1) is a lysosomal enzyme that plays a role in the degradation of lipid-modified proteins. PPT1 receives its catalytic power from its catalytic triad, the α/β hydrolase fold, and its hydrophobic groove in order to remove fatty acid acyl groups, typically palmitate from cysteine residues in proteins. PPT1 demonstrates how proteins can be modified by different enzymes and induce biological changes. Misregulation of PPT1 modifications can cause various diseases, including infantile neuronal ceroid lipofuscinosis, kufs disease, and late-infantile neuronal ceroid lipofuscinosis. Within these diseases, the production of PPT1 is decreased or eliminated completely, which leads to fatty acid buildup.
Structure
The secondary structure of PPT1 contains several α-helices and few β-sheets.
α/β Hydrolase Fold
The α/β Hydrolase Fold is common to many other hydrolases. This fold consists of β3-β8, αA, αB, αC, and αF.
Catalytic Triad
Hydrophobic Groove
Function
Biological
PPT1 is biologically involved in sensory transduction and the vision process.
Molecular
The main molecular function of PPT1 is to breakdown lipid-modified proteins.
Medical Relevance
Headline text
Headline text