| Structural highlights
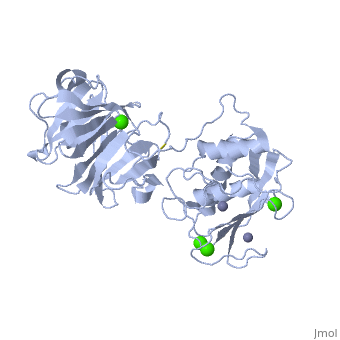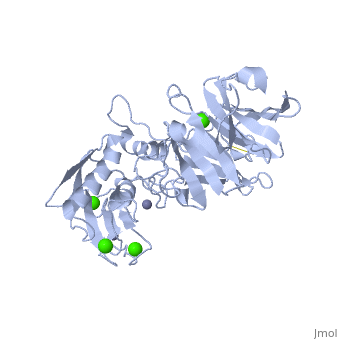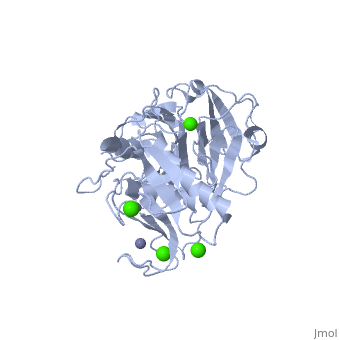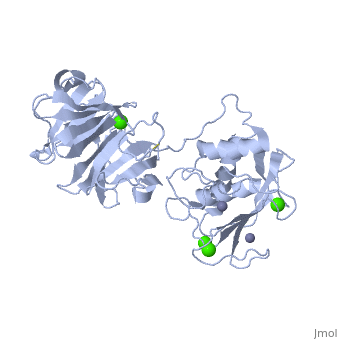
2clt is a 2 chain structure with sequence from Homo sapiens. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance.
| | Ligands: | ,
| | Related: | 1ayk, 1cge, 1cgf, 1cgl, 1hfc, 1su3, 2ayk, 2tcl, 3ayk, 4ayk, 966c |
| Activity: | Interstitial collagenase, with EC number 3.4.24.7 |
| Resources: | FirstGlance, OCA, RCSB, PDBsum |
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The extracellular matrix is a dynamic environment that constantly undergoes remodelling and degradation during vital physiological processes such as angiogenesis, wound healing, and development. Unbalanced extracellular matrix breakdown is associated with many diseases such as arthritis, cancer and fibrosis. Interstitial collagen is degraded by matrix metalloproteinases with collagenolytic activity by MMP-1, MMP-8 and MMP-13, collectively known as the collagenases. Matrix metalloproteinase 1 (MMP-1) plays a pivotal role in degradation of interstitial collagen types I, II, and III. Here, we report the crystal structure of the active form of human MMP-1 at 2.67 A resolution. This is the first MMP-1 structure that is free of inhibitor and a water molecule essential for peptide hydrolysis is observed coordinated with the active site zinc. Comparing this structure with the human proMMP-1 shows significant structural differences, mainly in the relative orientation of the hemopexin domain, between the pro form and active form of the human enzyme.
Crystal structure of an active form of human MMP-1.,Iyer S, Visse R, Nagase H, Acharya KR J Mol Biol. 2006 Sep 8;362(1):78-88. Epub 2006 Aug 4. PMID:16890240[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Iyer S, Visse R, Nagase H, Acharya KR. Crystal structure of an active form of human MMP-1. J Mol Biol. 2006 Sep 8;362(1):78-88. Epub 2006 Aug 4. PMID:16890240 doi:10.1016/j.jmb.2006.06.079
|