Sandbox Reserved 951
From Proteopedia
| |||||||||||
References
- ↑
Thus, this oxydo-reductase is involved in severals reactions.
Light emission
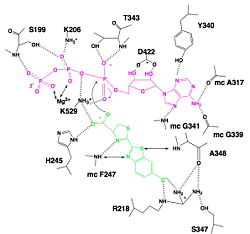
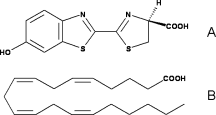
Of course, the most known reaction of luciferase is the light emission. In this reaction, luciferase firstly synthetized luciferin-AMP from luciferin and ATP, using a Mg2+ ion to offset the negative charges of the phosphate groups. Then, luciferase turns the luciferin-AMP into oxyluciferin in an excited state thanks to a dioxygen. This step releases AMP and CO2. The excited oxyluciferin relaxes and looses a photon so light is emitted. The wavelength of the light can vary with the pH : at the physiological pH, the emitted light is green and at a lower pH, the color is red.
Fatty-acyl-CoA synthesis
This reaction uses similar reaction but instead of taking luciferin as ligand, it takes fatty acids. Using ATP-Mg2+, it firstly forms fatty-acyl-AMP. And then, CoA-SH attacks the carboxylic group of fatty-acyl-AMP and so forms fatty-acyl-CoA.
Luciferin & Coenzyme A
It has been found that the reaction between luciferin and CoA is possible, forming in a first step luciferin-AMP and then luciferin-CoA. This reaction leads to an interesting biological phenomenon : when this reaction occurs in parallel of light emission reaction, we don't have a flash of light but a continuous light emission. This is because luciferin-AMP is a competitive inhibitor of the reaction whereas the luciferin-CoA is not. So the inhibition is deleted and the reaction continue to occur.<ref>PMID:18949818</li> <li id="cite_note-name-1">↑ <sup>[[#cite_ref-name_1-0|2.0]]</sup> <sup>[[#cite_ref-name_1-1|2.1]]</sup> <sup>[[#cite_ref-name_1-2|2.2]]</sup> <sup>[[#cite_ref-name_1-3|2.3]]</sup> <sup>[[#cite_ref-name_1-4|2.4]]</sup> <sup>[[#cite_ref-name_1-5|2.5]]</sup> Conti E, Franks NP, Brick P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure. 1996 Mar 15;4(3):287-98. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8805533 8805533]</li> <li id="cite_note-2">[[#cite_ref-2|↑]] [http://www.photobiology.info/ Photobiology]</li> <li id="cite_note-3">[[#cite_ref-3|↑]] [http://www.photobiology.info/ Photobiology]</li> <li id="cite_note-4">[[#cite_ref-4|↑]] [http://www.photobiology.info/ Photobiology]</li> <li id="cite_note-5">[[#cite_ref-5|↑]] Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009 Jan;61(1):6-17. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18949818 18949818] doi:[http://dx.doi.org/10.1002/iub.134 10.1002/iub.134]</li> <li id="cite_note-6">[[#cite_ref-6|↑]] Hosseinkhani S. Molecular enigma of multicolor bioluminescence of firefly luciferase. Cell Mol Life Sci. 2011 Apr;68(7):1167-82. doi: 10.1007/s00018-010-0607-0. Epub, 2010 Dec 28. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/21188462 21188462] doi:[http://dx.doi.org/10.1007/s00018-010-0607-0 http://dx.doi.org/10.1007/s00018-010-0607-0]</li>
<li id="cite_note-7">[[#cite_ref-7|↑]] Inouye S. Firefly luciferase: an adenylate-forming enzyme for multicatalytic functions. Cell Mol Life Sci. 2010 Feb;67(3):387-404. Epub 2009 Oct 27. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/19859663 19859663] doi:[http://dx.doi.org/10.1007/s00018-009-0170-8 10.1007/s00018-009-0170-8]</li></ol></ref>