Pheromone binding proteins (PBPs) are specialized members of the insect odorant-binding protein (OBP) super-family.
The main purpse in the dult moth's short life is reproduction. In fact, the male and female moth invest all of theire energy and resourses hoping to reach to the ultimate goal- mating. This long journy begins when the female moth releases a sex pheromone, usualy in specific hours in the night [3].
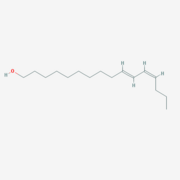
BmorPBP was first identified in the B. mori male antennae by Krieger et al. in 1996 [4], as the PBP of the first sex pheromone discovered ((E,Z)-10,12-hexadecadienol, or [Bombykol]). The male moth needs to detect minut amount of the pheromone in the air, while following turbulent wind-born pheromone trail and response fast (experimental evidence shows a response time of 0.5 seconds[5]). As the electrical signal transduction after the activation of the odorant receptor are too fast, Kaissling [6]have suggested that the
BmorPBP structure and function
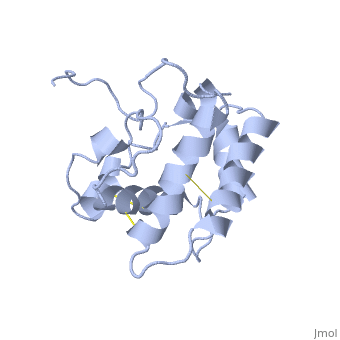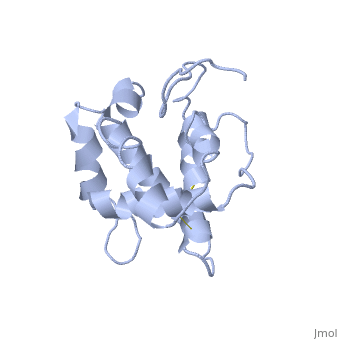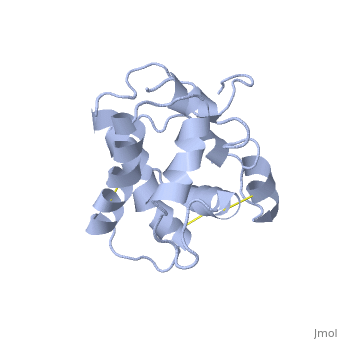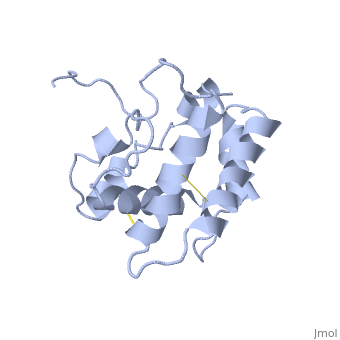
The protein has 164 amino acids that forms 6-7 alpha helices. Three disulfide bonds (in yellow) formed by residues tied four helices, and form the compact and robust sturcture of the protein. As expected from a soluble protein, its surface is covered with , which allows it to make interactions with the water molecule and sollubilize in the sensillar lymph.
BmorPBP ligand and ligand binding
The protein natural ligand is the moth pheromone . However, it was demonstrated that other molecules can also bound to the protein cavity [7]. The interaction with the ligand is beeing made by 4 alpha helices 1, 4, 5 and 6 in the core of the protein, which form the binding cavity [8].
Inside the binding cavity, are interacting with the pheromone, mainly by van der waals bounds. Out of those residues, some are conserved across OBP of lepidopteran (in green), and the rest are conserved in lepidopteran PBP only (in light blue).
In addition, the hydroxyl group of the pheromone bombykol forms a , Ser56 in red, oxygens are in purple (O–O distance of 2.8 Å).
Protein conformations
BmorPBP has two conformations: The "open form" (A) and the "close form" (B)[9]. The bombykol and the alpha-helix loacated in the c-terminus of the protein compete for the binding site: when the c-terminus is inside the binding cavity it get's an alpha helix shape, and the protien is in its "close form" (B), wherease in the "open form" (A) the c-terminus is outside of the protein and has no defined secondery sturcture. Binding experiments have shown that the B-form binds 15 times higher than the A-form [10], therefore considered to be the carrier of the pheromone. The complex of the A-form and the pheromone, is then considered the form that activates the receptor.
In a nuetral pH (6.5-7) the protein is in the "open form" (A), in which the
The transition between the two conformation is taking place between 6-5 pH.
The C terminus of the protein bears mostly nonpolar amino acids. Yet on the surface of the helix there are three exceptional amino acids: Asp-132, Glu-137, and Glu-141, which are conserved in moth PBP [11]. Of these, residues Asp-132 and Glu-141 triggers the formation of the α-helix upon protonation at low pH. This what is causing the ejacullate of the ligand from the binding pocket, which is replaced by the formated alpha helix[12].
The is both pH and ligand dependent [13][14][15].
Studies on other Lepidopterans that show a simmilar pH dependent conforamation suggests that this model is a genral model moth PBP[1].
Nonetheless, the enourmas diversity among insects is not allowing us to assume this model is true for all insects' OBPs.
Receptor activation
Two theories have been propsed for the activation of the odorant receptors located on the dendrtirte membrane. One theory suggests that the pheromone-PBP complex is needed for the receptor activation, while the second theory argue that the pheromone itself is sufficient for the activation of the receptor.
Image:Nmodel.png The events prior the neuron exsitation, according to "N model" suggested by Kaissling (2009)
[6] - Activation by the pheromone alone
This model is supported by the pH dependent conforamtion transition, that is described above.
- Activation by the complex pheromone-PBP
An alternative mode action was proposed for the receptor activation in the drosophila Xu P, Atkinson R, Jones DN, Smith DP. 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45:193–200.
Laughlin JD, Ha TS, Jones DNM, Smith DP. 2008. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 133:1255–65
The surface of the dendrite is negatively charged [16], which cause the accumulation of positively charged kations near the membrane surface (20-50 nm), thereby inducing a low pH environment near the dendrite membrane [6].