This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Salicylate synthase from Mycobacterim tuberculosis (MtbI) is a highly promiscuous enzyme that has four distinct activities in vivo: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM). MtbI belongs to the chorismate-utilising enzyme family, which consists of structural homologues (, , , and ) that isomerize chromate to isochorismate. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis [3]. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation. IS, IPL, and SS activity are also modulated by the pH of the medium. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8[4]. The pH dependent activity of MbtI is related to the ionization state of the active site residues involved in the molecular mechanisms used by the enzyme to catalyze the different reactions[5].
The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in Mycobacterium tuberculosis(Figure 3)[6]. This complex secondary metabolite is essential for both virulence and survival of M. tuberculosis. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action.
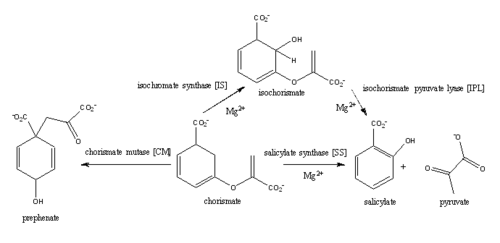
Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer
et al., 2012
Structure
Image:Active site cleft.png Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.
The crystal asymmetric unit was found to contain , however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes. The core of the protein is formed by 21 folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 (Figure 2).
Disease
Mycobacterium tuberculosis is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide.(CDC) Although various treatments for TB infection and TB disease exist, the emergence of multi-drug and extensively-drug resistant strains of M. tuberculosis has increased the need for anti-tubercular agents with novel modes of action. Iron is essential for mycobacterial growth and pathogenesis, M. tuberculosis obtains iron through mycobactin T, a type of siderophore. Mycobactin T is a hydrophilic molecule that has been proposed to be secreted actively into the aqueous growth medium for direct competition with iron-binding molecules of the environment. The core of the Mycobactin T siderophore is derived from salicylic acid which is synthesized from chorismate by salicylate synthase. The salicylate synthase activity of MbtI produces salicylate from chorismate through an isochorismate intermediate. This reaction is Mg2+ dependent. Chorismate is then used in the biosyntheses of Mycobactin T, the siderophore of M. tuberculosis. The siderophore sequesters iron, covering pathogenesis of M. tuberculosis.

Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway.
Function
Relevance
Structural highlights
Molecular Mechanism
Magnesium cation effect
The presence of the magnesium ion induces changes in the structure of the active site and in the substrate, as well as significant pKa shifts in some of the key residues involved in the catalytic activity.
The coordination shell of the magnesium cation in the active site of MbtI is composed of two water molecules, Glu434, Glu294, and the two oxygen atoms of the C1 carboxylate group of chorismate. A comparative analysis of the structure of the active site of MbtI with and without the magnesium ion
The charged magnesium cation displaces the positively charged Lys293 away from the active site while the negatively charged Glu297 is faced toward the active site.
changes in the chorismate molecule
pre-organizing the active site and shifting the pKa values of the amino acids.
Isochorismate pyruvae lyase (IPL)
For IPL activity, a proposed histidine residue (his334) acts as a base, abstracting the C2 proton of isochorismate through a second order elimination mechanism. The C9 atom of isochorismate is then protonated, leading to the elimination of pyruvate and formation of salicylate. At pH values below 7.5 the histidine residue is protonated and therefore not able to act as a base in the mechanism, causing an accumulation of isochorismate.
Isochorismate synthast (IS)
The C1 carboxylate group of chorismate binds to the magnesium cation within the active site.
Currently, isochorismate is believed to be formed from chorismate through a proposed Sn2 mechanism involving nucleophilic attack of an activated water molecule to the C2 center followed by either a concerted or stepwise elimination of the C4 hydroxyl group(He et al.). Lys205 has been proposed to act as the catalytic base, activating a water molecule in the active site by abstracting one of its protons. However, mutational analysis of Lys205 suggested that the lysine reside is not the sole determinant in the activation of a water molecule for nucleophilic attack of the C2 center. Studies have shown that Lys205 is protonated at neutral pH and therefore can't act as a base to activate the water molecule, agreeing with the mutational analysis data. The magnesium ion also causes the Lys205 residue is also displaced away from the active site. Instead of Lys205, Glu297 residue has been proposed to act as a base in the activation of the water molecule. The magnesium ion forces the negatively charged Glu297 residue to face toward the active site and the pKa of Glu297 (3.9) suggest an unprotonated state. Furthermore, Glu297 forms a hydrogen bond with a water molecule within the active site as well as with Lys205, which is in turn hydrogen bonded to C1 carboxylate group of chorismate and the oxygen of the nucleophilic water molecule. The glutamic residue (Gly252) could protonate the C4 leaving hydroxyl group. The pKa of Gly252 (7.7) suggest that is it is the only protonated glutamate residue in the active site at pH 7 and thus able to protonate the C4 leaving group. The pKa of Gly252 also accounts for the accumulation of isochorismate at pH values below 7.5.
isochorismate pyruvate lyase (IPL)
salicylate synthase (SS)
chorismate mutase (CM)
A magnesium ion in the active site orients the C1 carboxyl group of chorismate. A lysine residue then serves as a general base for the activation of a water molecule to attack at C2
The catalytic mechanism for conversion of isochorismate to salicylate by MbtI is a sigmatropic, pericyclic mechanism that is pH-dependent. Chromate mutase activity is only observed in the absence of magnesium ion in the active site while salicylate synthase activity is depended on magnesium ion. The active site of MbtI is altered by the removal of the magnesium cofactor causing chromate mutase activity. MbtI has differing binding modes for chromate that leads to different substrate conformations/transition states and resulting in different products.
Inhibition Studies
MbtI Inhibition studies aid in the future design of anti-tubercular agents and broad-spectrum antibiotics. Mimics of the enzyme-bound intermediate of MbtI, isochorismate, prove to be significantly more potent inhibitors than the substrate, chorismate mimics (Alexandra Manos-Turve, 2010). Specifically, 2-hydroxybenzoate-based inhibitors that contain extended hydrophobic enrol ether side chains at C3 in place of the enos-pyruvate side chain found in chorismate and isochorismate.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.


