Sandbox Reserved 1063
From Proteopedia
FadD13
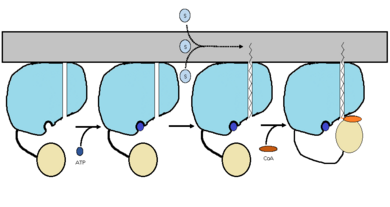
IntroductionMycobacterium Tuberculosis is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate lipids and fatty acids before going into metabolic pathways. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Shown below is the general mechanism for ACS proteins. Image:FadD13 steps.jpg Figure 1 Figure 1 shows the general outline of the binding of ATP and acyl substrates to an ACSVL enzyme. This is the accepted mechanism for these types of proteins. BackgroundMycobacterium Tuberculosis is the causative agent of Tuberculosis commonly abbreviated TB. TB causes approximately 1.4 million deaths every year. The cost for treatment of patients with TB between the years 2010-2015 was approximately 16 billion dollars. TB is spread through the air, not by contact. There are two forms of TB, latent TB and TB disease. Structural HighlightsFadD13 is an ACSVL enzyme that can accept lipids up to 26 carbons as well as being a peripheral-membrane proteins. Unlike other ACSVL proteins, FadD13 is soluble. There are numerous aspects of its structure that affects the way this protein functions. There are two domains of this protein, a larger N-terminal domain and a smaller C-terminal domain. These domains are held together by a six amino acid linker. Inside the larger N-terminal domain is a hydrophobic tunnel, which allows large lipids/fatty acids, up to 26 carbons, to bind. The tunnel is capped by an arginine-rich lid loop that is involved in the peripheral binding of the enzyme to the membrane. These structural features The first important structural point of note is that there are two subunits, the larger N-terminal subunit () and the smaller C-terminal subunit () held together by a six amino acid linker ().The allows for either ATP or AMP to bind and activate FadD13. The next major region of note is the hydrophobic tunnel which allows for lipid binding up to 26 carbons in length and extends from the ATP and AMP binding region. The hydrophobic tunnel is capped by an arginine and aromatic-rich surface patch which is involved in membrane binding of the protein[1].Three key arginine residues, all play an important role for the enzyme to be able to bind to the cell wall. FunctionThe FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the . Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From there CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins[2].
| ||||||||||||