Introduction
Phospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate [IP2] to the second messengers inositol 1,4,5-trisphosphate [IP3] and diacylglycerol [DAG] in an essential step for the physiological action of many hormones, neurotransmitters, growth factors, and other extracellular stimuli. These cascades use signaling complexes consisting of G alpha subunits of the Gq family of heterotrimeric guanine nucleotide–binding proteins (G proteins) and PLC-beta isozymes (β1-4). Agonist-stimulated receptors increase exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Gαq. GTP-bound Gαq engages and activates PLC- β3, and PLC- β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism when the PLC-β3 enzyme has the ability to terminate the Gαq protein signal in addition to being activated by it.[1] [2]
Structural highlights
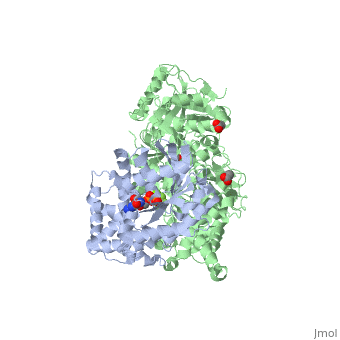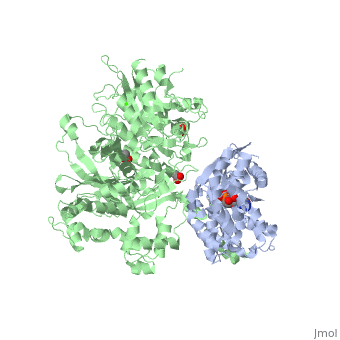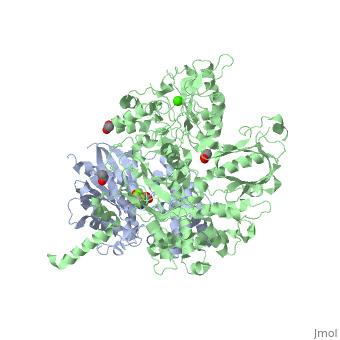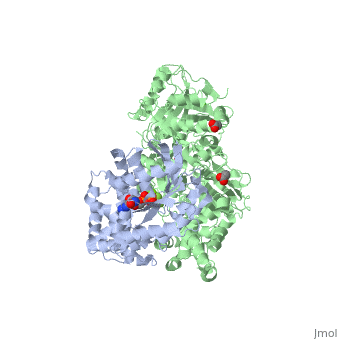
The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called switch regions I-III.
consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel and a C2 domain.
PLC- β3 engages Gαq throughout three regions. First, an extended loop between the third and fourth EF hands of PLC- β3 directly buttresses switch residues critical for GTP hydrolysis by Gαq. Second, the region of PLC- β3 that connects the catalytic TIM barrel and the C2 domain interacts with both switches 1 and 2 of Gαq. Third, a segment composed of a helix-turn-helix at the C terminus of the C2 domain resides primarily within a shallow declivity on the surface of Gαq formed by switch 2 and α3.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.