Introduction
Phospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate [IP2] to the second messengers inositol 1,4,5-trisphosphate [IP3] and diacylglycerol [DAG] in an essential step for many physiological cascades. When the receptor is stimulated by ligand of some kind it increase exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Gαq. GTP-bound Gαq activates PLC- β3, and PLC- β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism when the PLC-β3 enzyme has the ability to terminate the Gαq protein signal in addition to being activated by it.[1] [2]
Structural highlights
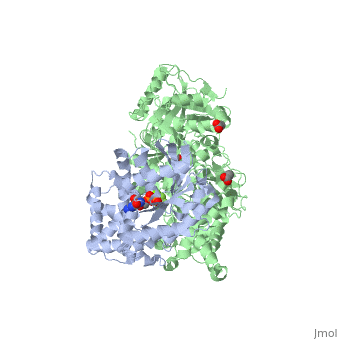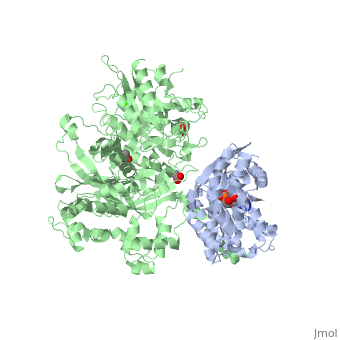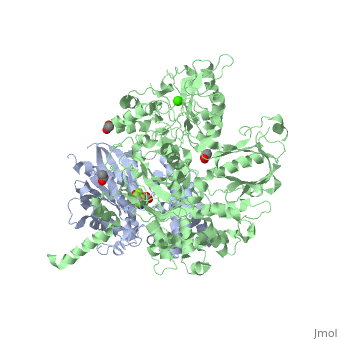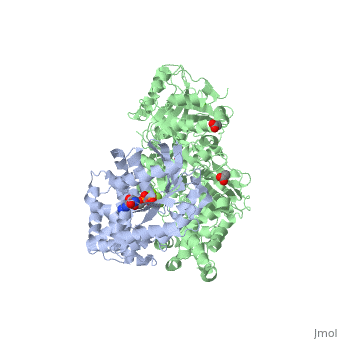
The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called .
consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel that the X/Y linker connect its two halves and a C2 domain.
PLC- β3 engages Gαq throughout three regions. First, an extended loop between the third and fourth EF hands of PLC- β3 directly supports switch residues critical for GTP hydrolysis by Gαq. Second, the region of PLC- β3 that connects the catalytic TIM barrel and the C2 domain interacts with both switches 1 and 2 of Gαq. Third, a segment composed of a helix-turn-helix at the C terminus of the C2 domain mostly located within a shallow declivity on the surface of Gαq formed by switch 2 and α3.
PLC-β3 interacts with a surface on Gαq that with portions of Gα subunits needed for engagement of RGS proteins and the effector-binding region. Other effectors are known to engage the within Gα subunits. There are a large family of regulator of G protein signaling (RGS) proteins that independently accelerates the GTP hydrolysis in the .