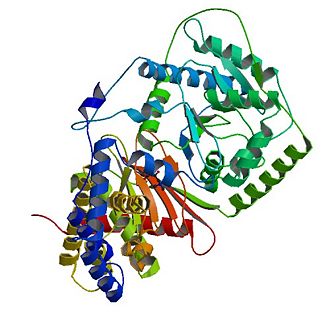
Carnitine acetyltransferase
From Proteopedia
| |||||||||||
Contents |
Catalytic Mechanism of Carnitine Acyltransferases
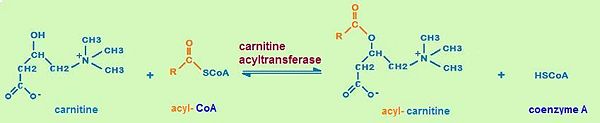
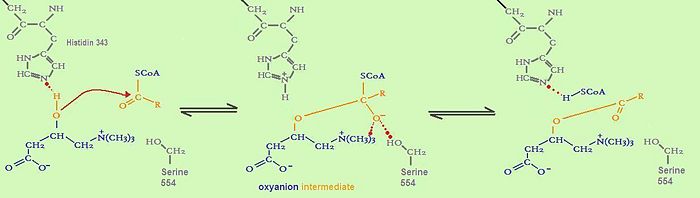
It is assumed that the whole family of carnitine acyltransferases share the same catalytic mechanism, because certain residues in the catalytic side (histidine343, serine554) are conserved throughout the family. Histidine 343 is probably the most important residue in catalysis. First, it induces optimal substrate binding by forming a hydrogen bond between its side chain and the hydrogen atom of the substrate’s reactive group. As soon as all substrates attained the right position, the catalytic histidine residue is ready to extract a proton from either the hydroxyl group of carnitine or the thiol group of CoA. The catalytic histidine residue can be considered as a general base in catalysis.Which proton is extracted depends on the direction of the reaction. Acyl- carnitine is formed by extracting a proton from carnitine, whereas acyl-CoA is formed by extracting a proton from CoA.
The extraction of a hydrogen atom leads to the development of a tetrahedral oxyanion intermediate. This oxyanion is stabilized by the side chain hydroxyl of serine 554 through hydrogen bonding as well as by the positive charge on the trimethylammonium group of carnitine. As the positive charge of the carnitine substrate is necessary for the carnitine acyltransferase mediated reaction to happen, this catalysis can be described as substrate-assisted catalysis.[4]
Regulation
One of the most common regulation systems of carnitine acyltransferases involves inhibition by malonyl-CoA, an intermediate in the synthesis of fatty acids. Malonyl-CoA inhibits long-chain carnitine acyltransferase activity by all three enzymes at similar concentrations in the physiological range. Moreover, the mitochondrial (CAT) and peroxisomal (COT) enzymes can also be regulated through mRNA transcription by a number of shared factors. Although the microsomal enzyme is less well studied, there does, indeed, appear to be a pattern of coordinate regulation for this system.
Carnitine acetyltransferase deficiency and diseases
Mutation and dysregulation of CPTs are linked to serious human diseases. Recessive mutations of CPT-I and CPT-IICPT-I and CPT-II are crucial for the beta-oxidation of long-chain fatty acids in the mitochondria by enabling their transport across the mitochondrial membrane. can produce hypoketonemia and hypoglycemia in patients, while CPT-II deficiency is the most common cause of abnormal lipid metabolism in skeletal muscle.Single-point mutations as well as insertions/deletions in the CPT genes can produce the clinical phenotype. The hypoglycemia observed in patients with reduced CPT-I activity suggests that antagonists of CPT-Is may be able to lower blood glucose levels. A covalent CPT-I inhibitor, etomoxir, can lower blood glucose levels in diabetic animals and humans, showing that such inhibitors may be efficacious for the treatment of type 2 diabetes.
3D structures of carnitine acetyltransferase
Updated on 27-May-2015
1ndb – mCAT – mouse
1t7n – mCAT (mutant)
1ndf – mCAT + carnitine
1t7o – mCAT (mutant) + carnitine
2h3u – mCAT + carnitine + CoA
1t7q – mCAT (mutant) + carnitine + CoA
2h3w – mCAT (mutant) + hexanoylcarnitine + CoA
2h3p – mCAT + Carnitine + acetyl-CoA
1ndi – mCAT + CoA
1s5o – hCAT + carnitine - human
1nm8 – hCAT
References
- ↑ 1.0 1.1 Donghai Wu‡, Lakshmanan Govindasamy§, Wei Lian‡, Yunrong Gu‡, Thomas Kukar‡,Mavis Agbandje-McKenna§, and Robert McKenna§¶.Structure of Human Carnitine Acetyltransferase.Published, JBC Papers in Press, January 31, 2003 DOI 10.1074/jbc.M21235620
- ↑ 2.0 2.1 2.2 Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003 Jan 10;112(1):113-22. PMID:12526798
- ↑ Lehninger Principles of Biochemistry |ISBN-10: 071677108X | ISBN-13: 978-0716771081 | Publication Date: February 1, 2008 | Edition: 5th
- ↑ 4.0 4.1 Jogl G, Hsiao YS, Tong L. Structure and function of carnitine acyltransferases. Ann N Y Acad Sci. 2004 Nov;1033:17-29. PMID:15591000 doi:10.1196/annals.1320.002
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Alina Spielmann, Ndeye Coumba