Flippases are the homodimeric transmembrane proteins located in the membrane. It helps phospholipid to transport from inner face to outer face of the cell membrane. It is composed with alpha-helix and beta sheets. The main three structural features of each monomer are an external helix, a belt of positively charged amino acids, and a binding site for an ATP molecule.
This is a default text for your page Sandbox flippases. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Structure
Flippase is homodimer which is quartinary strucure. Each monomer is tertiary structure composed with nine alpha-helices and five beta sheets. The secondary structure, helix is composed with amino acids which are primary structure. The main three structural features of each monomer are an external helix, a belt of positively charged amino acids, and a binding site for an ATP molecule. External helix is hydrophobic groove and it is composed with amino acids(K55,Y63,Y56,R53,D47,Y50,T43). A positively charged belt has four Arginines(R302,R86,R260,R309) which are negative charged.
Function
Flippases flip the lipid from cytoplasmic(in) side to periplasmic(out) side.
Mechanism
The beginning conformation is an outward-occluded conformation and the head of Linked-lipid Oligosaccharide(LLO) is on the cytoplasmic side. When the tail of LLO is attached to external helix, the ADP exchanges to ATP and the conformation changes to outward-open state. The head of LLO attaches to a positively charged belt facing to periplasmic side since the pyrophosphate has negative charge which attracts to positive charge. As ATP changes to ADP, the conformation returns to outward-occluded conformation and it squeezes the LLO head out to periplasmic side of the membrane and the tail of LLO is released to extend to cytoplasmic side. Therefore, the position of LLO is opposite through this mechanism. [3]
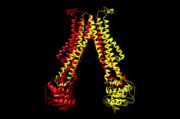
5c76, outward-open conformation
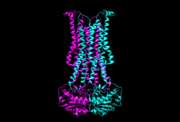
5c73, outward-occluded conformation
Energetic
As the tail of LLO is attached to external helix, it acts as the switch for the ATPase to supply the ATP.[4]
Disease
Relevance
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.


