Human BCL-2, isoform 1 is an oncoprotein of 239 residues regulating cell death (apoptosis), notably acting as an anti-apoptotic. It is encoded by the BCL2 gene located on the 18th chromosome (63.12-63.32 Mb). There are 2 isoforms of this protein (𝛼 and 𝛽), produced by alternative splicing, and which differ by 2 aminoacids (residues 96 (A→T) and 110 (R→G))(ref). Alteration of this protein is a caused of many cancers, and is also likely to be involved in schyzophrenia and autoimmunity.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Primary & Secondary Structure
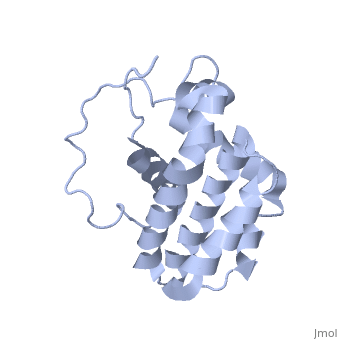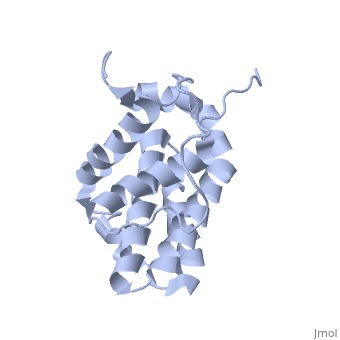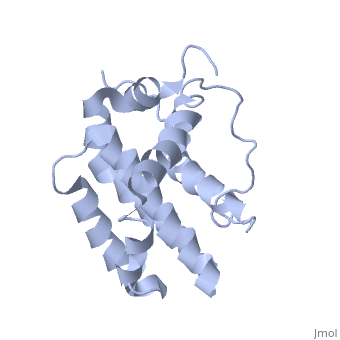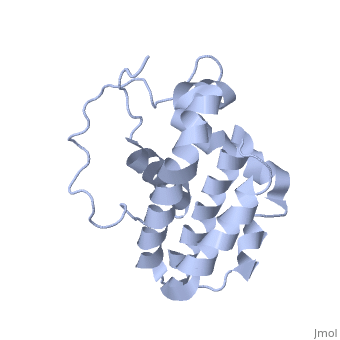
Human BCL-2, isoform 1 is a 26kDa protein of 239 residues. The linear structure highlights 5 domains: (10-30),
(93-107), BH1 (136-155), BH2 (187-202) and a transmembrane domain (218-239). It organizes as eight alpha-helices: from 11 to 25 (1) , from 93 to 107 (2), from 109 to 118 (3), from 126 to 137 (4), from 144-163 (5), from 169 to 184 (6), from 186 to 191 (7) and from 194 to 202(8). There are also 3 turns (32-34, 123-125, 138-140). The 3rd alpha-helix is a 3(10) helix, whereas BCL-XL 3rd helix is a normal alpha-helix.
Function
IP3R inhibition
Bcl-2 localized at the endoplasmic reticulum (ER) membranes participates in the control of Ca2+ content and release. The inositol 1,4,5-trisphosphate receptor (IP3R) which is the primary Ca2+ release channel localized in the ER. Its pro-apoptotic activity can be directly inhibited by the Bcl-2, homology domain 4 (BH4) being essential and sufficient for this effect. BH4 comprises 20 amino acids (10-30) organized in alpha-helical structure which is required to inhibit IP3R. Residues K17, H20, Y21 and R26 participate in the inhibition of IP3R because they are very accessible and proximal in the secondary structure. [3]
Regulation of the mitochondrial pathway of apoptosis
BH3-only proteins which belong to the Bcl-2 family activate pro-apoptotic proteins such as Bcl-2-associated X protein (Bax) or Bcl-2 antagonist/killer-1 (Bak) at the mitochondrion. When Bax or Bak are activated, they homo-oligomerize and form the pores in the outer mitochondrial membrane which are necessary for the pro-apoptotic molecules (including second mitochondria-derived activator of caspase and cytochrome c) to escape. Then cytochrome c leads to the activation of caspases which are actually proteases that degrade the key proteins of the cell.
On the other hand, Bcl-2 may prevent the activation and homo-oligomerization of Bax and Bak thus blocking the cell death. This is achieved by sequestering BH3-only proteins or activated and monomeric Bax and Bak. [4]
Therefore, the neutralization of Bcl-2 is required for eficient cell-death. BH3 proteins Bad, Bim and Puma bind Bcl-2 and disable its anti-apoptotic activity. BH3 peptides occupy the hydrophobic pocket of Bcl-2 and the sequestered pro-apoptotic proteins are released. [5] [6]
Bcl-2 can also inhibit the release of cytochrome c from mitochondria by preventing the mitochondrial depolarization. [7]
Disease
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.