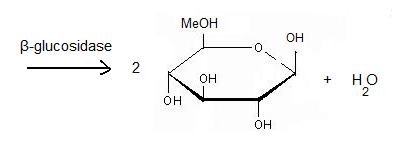
β-glucosidase is an enzyme which catalyses the hydrolysis of terminal non-reducing residues in β-glucosides (EC number : 3.2.1.21). In the case of 2VRJ, it comes from Thermotoga maritima which is a rod-shaped bacterium belonging to the order of Thermotogates. This bacterium was originally isolated from geothermal heated marine sediments.
2VRJ is here is in complex with an inhibitor called N-octyl-5-deoxy66-oxa-N-carbamoylcalystegine [1]. Raucaffricine β-glucosidase (RGB) catalyzes the conversion of raucaffricine to glucose and vomilenine. Some more details in Molecular Playground/Beta-galactosidase.
General action as biocatalyst
It acts on the β(1-4) bond linking two glucose residues or glucose-substituted molecules. The action of the enzyme on such glucosides results in the release of units of glucose. For instance, hydrolysis of cellobiose catalysed by a β-glucosidase releases two glucoses [2].
β-glucosidases can also be called β-D-glucoside glucohydrolases or cellobiases.
2VRJ
Structure and function
In terms of structure 2VRJ is a homodimer. It means that it is composed of two chains and which are chiral. Each chain is composed of 438 residues and constitutes a subunit of the protein. Each subunit contains a catalytic site.
The enzymatic hydrolysis of a glycosidic bond requires two critical residues : a proton donor and a proton acceptor which can also be called a nucleophile/base. Aspartate and glutamate have been found to perform catalysis [3]. Accorded to this, studies showed that one of the conserved regions of β-glucosidases is centred on conserved glutamate residues [4].
As every β-glucosidase, 2VRJ presents two conserved residues of glutamate (). Moreover 2VRJ has a third important residue : [5].
The protein is presented in complex with an inhibitor called . We can see that the two glutamate residues and the asparagin are really closed to each other and to the . Such a proximity highly suggests that there are important interactions between them. So we can say that the catalytic site of 2VRJ is composed of two glutamate and one asparagin.
There are three different topologies for the active site of β-glucosidases : the pocket or crater, the cleft or groove and the tunnel [6]. The topology of 2VRJ active site is a in which the ligand can bind.
Hydrolysis of terminal non-reducing residues in β-glucosides
There are two ways to hydrolyse the terminal non-reducing residues in β-glucosides which implicate the two glutamate residues and a molecule of water [7]. Water which is an amphoter, is here used as a base for the nucleophilic attack on the positively charged anomeric carbon.
The general equation of the chemical reaction is :
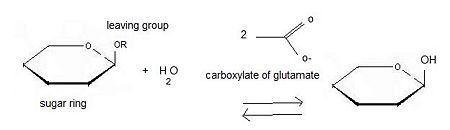
Inverting glycoside hydrolases
Inverting glycoside hydrolases lead to an inversion of the anomeric configuration to create an alpha configuration. The steps of the reaction are like the mechanism of nucleophilic substitution S2N. It is an one step process: the nucleophile (water) the anomeric carbon with simultaneous expulsion of the leaving group (OR). Bond making takes place at the same time as bond breaking. Such a mechanism is called concerted reaction.
The distance between the two carboxylates is about 10.5 angströms.
Retaining glycoside hydrolases
Retaining glycoside hydrolyses occur in two steps:
the first step, called glycosylation leads to the release of the leaving group and the creation of a carbocation. Subsequently, water attacks this last one.
The second step, called deglycosylation consists of OR- nucleophilic attack on the intermediate and allows the deglycosylation of the enzyme.
In this case, there are two transition states involved.
The distance between the two carboxylates for this mechanism is about 5.5 angströms.
For 2VRJ the distance between its two glutamates is about angströms. So we can say that 2VRJ seems to be a retaining enzyme.
NB: The values of the pH and the nature of the solvent play a main role in the rate of the reaction.
Glutamates are directly involved in the catalytic reaction but asparagine is used to stabilise the structure.
Other use of β-glucosidases
β-glucosidase is now used for the synthesis of biofuel. Wood is an abundant and renewable energy which can be changed into bioethanol thanks to enzymatic hydrolysis. This synthesis needs five steps. First it is pre-hydrolysis. The structure is divided into lignin and (hemi)cellulose. The cellulase can better access the structure to act on it.
The second step: hydrolysis is the most important. A cellulase is a complex of 3 enzymes which act together to hydrolyse cellulose: Endoglucanase breaks the chain in the middle of the molecular structure of cellulose. Exoglucanase binds an available end of the chain and isolates it. Then units of cellobiose are cut (two units of glucose which are together). To finish, β-glucosidase divides cellobiose into two glucoses. When they ferment, they become ethanol. The final product is obtained thanks to fermentation, distillation and deshydratation.
Human acid-beta-glucosidase covalently bound to conduritol B epoxide
The crystal structure of the human acid-β-glucosidase (acid-beta-glucosidase, glucocerebrosidase, , E.C. 3.2.1.45, colored yellow with covalently bound irreversible inhibitor (conduritol-B-epoxide; CBE; shown in cyan with its hydroxyl groups are in red) was solved. This structure reveals that binding of CBE to the active site does not induce a global conformational change in GlcCerase and confirms that Glu340 is the active-site catalytic nucleophile, because the between the cyclohexitol C1 atom and Glu340 Oε2 is 1.43 Å. The comparison between the active sites of and another representative of the glycohydrolase family - plant (1iev), reveals that CBE bound with this plant enzyme adopted the "chair" conformation, while with human , it is observed in a "boat" conformation, with hydrogen bonds to Asn234 Oδ1 and Nδ2, Glu340 Oε1, Trp179 Nε1, and Asp127 Oδ1 and Oδ2.
Only one of two of a pair of flexible loops (L1: Ser345–Glu349, and L2: Val394–Asp399) located at the entrance to the active site in native GlcCerase (1ogs) is observed in the GlcCerase-CBE structure, a conformation in which the active site is accessible to CBE (colored blue), while these loops in the second (closed) conformation are colored magenta. In , a major structural change is observed in the positions of , and in a more limited difference is observed in the conformations of . Analysis of the dynamics of these two alternative conformations suggests that the two loops act as a lid at the entrance to the active site. The movies 1 and 2 illustrate the dynamics of the movement of these two loops.
Additional Resources
For additional information, see: Carbohydrate Metabolism