Function
Heat shock factor (HSF) are transcriptional activators of heat shock genes. HSF bind to heat shock sequence elements throughout the genome with a consensus array of three oppositely oriented sequence AGGAN and activate transcription. Each HSF monomer contains one C-terminal and 3 N-terminal leucine zippers. Two sequences flanking the N-terminal leucine zippers contain the consensus nuclear localization signal (NLS). The DNA-binding domain (DBD) of HSF lies in the N-terminal of the first NLS region[1].
Structural highlights
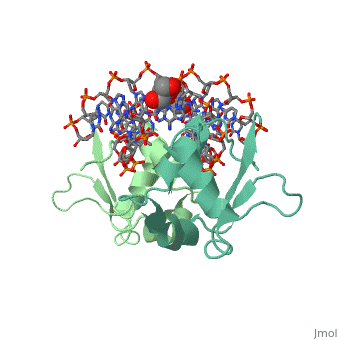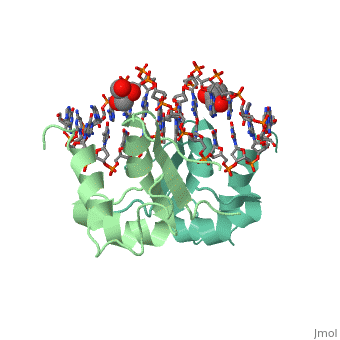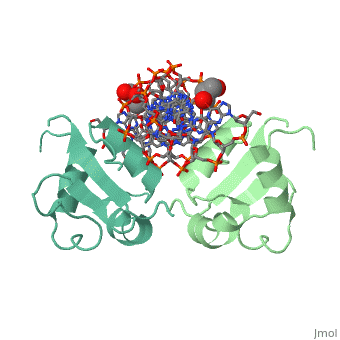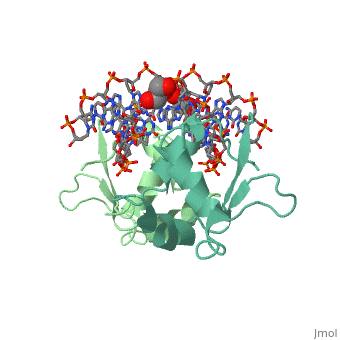
The DBD region of HSF shows multiple protein-DNA interactions as well as water-mediated interactions [2].
3D structures of heat shock factor
Updated on 20-March-2016
1hks, 1hkt – HSF DBD – Drosophila melanogaster – NMR
2ldu – HSF DBD – human - NMR
2hts, 1fbu – KlHSF DBD – Kluyveromyces lactis
3htf, 1fbq, 1fbs – KlHSF DBD (mutant)
3hts – KlHSF DBD + DNA
1fyk, 1fyl, 1fym – KlHSF DBD (mutant) + DNA
References
- ↑ Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993 Mar;13(3):1392-407. PMID:8441385
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269