metabotropic Glutamate Receptor 5 Homo sapiens
Introduction
G-coupled protein receptors GPCR are trans-membrane proteins that are integral to cell signaling. The human genome encodes for approximately 750 GPCRS, 350 of which are known to respond to extracellular ligands.[1]. GPCRs are divided into four major classes based on sequence similarity and transduction mechanism; Class A,B,C, and F.[2]. Metabotropic Glutamate Receptor 5 (mGlu5) is a class C GPCR that is involved in the Gq pathway [3]. mGlu5is highly expressed in neuronal and glial cells in the central nervous system, where glutamate serves as the major neurotransmitter. When glutamate binds to the extracellular domain of mGlu5, consisting of the Venus Fly Trap [4], a conformational change through the tram-membrane domains activates the coupled G-protein. This G-protein disassociates and the alpha subunit activates Phospholipase C which has the final outcome of increased neuronal activity[2].
Structure
Overall Stucture
mGlu5 is seen as a homodimer in vivo, with each subunit comprising three domains; extracellular, trans-membrane and cysteine-rich. The structures shown here are centered on the trans-membrane domain, comprised of seven α-helices all roughly parallel to one another [4]. Also displayed are Intracellular Loop (ICL) 1 forming a short α-helix, ICL3 and Extracellular Loops (ECL) 1 and 3, which lack secondary structure, and ECL2, which interacts with trans-membrane (TM) helices 1,2 and 3 and ECL 1[4].
Key Interactions
Clinical Relevance
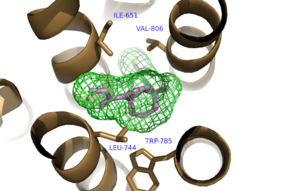
Hydrophobic Pocket Surrounding Mavoglurant
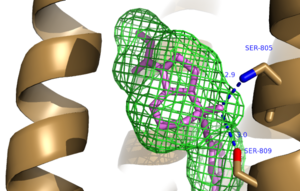
Further Hydrogen Bonding between protein and Mavoglurant


