This is a default text for your page Nos1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Disease
Nitric oxide (NO) is produced from one of three synthases present within the body: neuronal nitric oxide synthase 1 (NOS1), inducible nitric oxide synthase 2 (NOS2), and endothelial nitric oxide synthase 3 (NOS3) (Shinkai, et. al, 2002). Specifically, NOS1 has nine different first exons that lead to multiple NOS1 transcripts with different 5’-untranslated regions (Galimberti, et. al, 2008). An advantage of having multiple first exons that can be alternatively spliced and expressed is that NOS1 can be specific and specifically regulated for different tissues (Galimberti, et. al, 2008). A disadvantage to having multiple first exons is that there is a higher possibility of mutations, which would affect NO production and thus have an effect on the second messenger cyclic guanine monophosphate (cGMP) production (Shinkai, et. al, 2002). Furthermore, because NO is an oxyradical, overproduction caused by a mutation can lead to neural tissue damage (Shinkai, et. al, 2002). Numerous pathologies may arise from neural tissue damage, and one study suggested overproduction of NO that leads to such damage is possibly an influential factor in developing schizophrenia (Shinkai, et. al, 2002).
Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009).
Relevance
Structural highlights
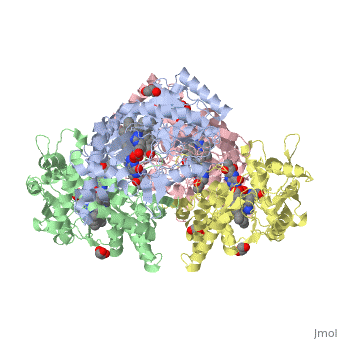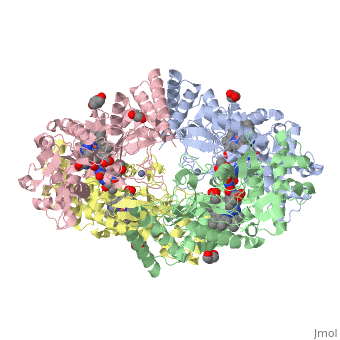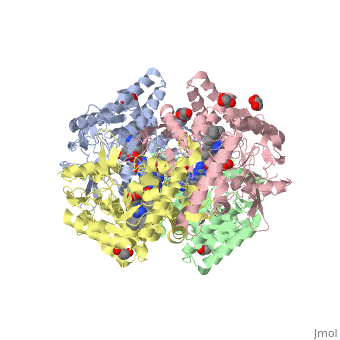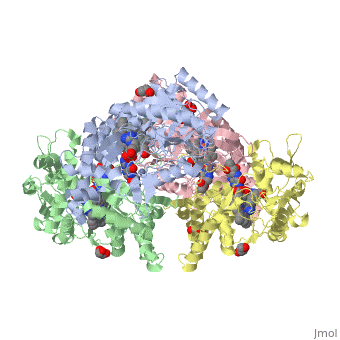
Nos exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme and pterin. Nos has a N-terminal catalytic domain with a heme active site. Nos 1 also has a cofactor site nearby for tetrahydrobiopterin (H4B). Nos has a C-terminal reductase domain containing FMN, FAD and NADPH binding sites. Electrons are passed from FAD to FMN and then to the heme. This electron flow is controlled by the binding of CaM and Ca2+ in the linker region between the two major domains. The linker between the oxygenase and reductase domains contains a calmodulin-binding sequence. Nos1 is found in neuronal tissue.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.