Sandbox WWC7
From Proteopedia
Pentatricopeptide repeat (PPR) proteins are a family of sequence specific RNA-binding proteins which participate in organelle RNA metabolism. Although the mechanisms of RNA binding and the functions of PPR proteins are not fully understood, PPR proteins are thought to assist in RNA editing,[1] translation,[2] and organelle biogenesis.[3] They make up the majority of RNA-binding factors in plant organelles. PPR proteins are characterized by a series of tandem-repeat amino acid consensus sequences which form α-helix . These hairpin structures accumulate to form a α-solenoid tertiary structure. PPR proteins belong to one of two classes: P-class and PLS-class, with the P-class containing 35 amino acid repeats and the PLS-class containing 31-36 amino acid repeats. PPR10 is a well-characterized P-class PPR protein found in the chloroplast of Zea mays.[2]
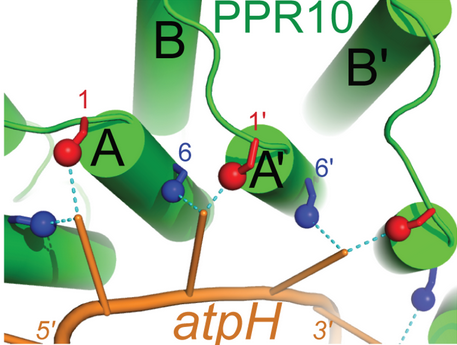
FunctionIn the Zea mays plastid, PPR10 binds specifically to the ssRNA oligonucleotides ATPH (17 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') and SPAJ (18 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') where it has been shown to shield the transcripts from ribonucleases. In addition to stabilizing these RNA sequences, PPR10 increases the rate at which they are translated.[2] MechanismThe primary factor in the ability of PPR10 to bind RNA bases in a modular fashion lies in the identities of the residue at position 6 on a repeat and the residue at position 1 on the next repeat. For example, in the structure to the right, Through van der Waals interactions, Val210 and Arg175 (both orange) also contribute to the specific binding of guanine in this example. These residues force G1 into a conformation where it forms a hydrogen bond to Thr178. The example of PPR10 binding G1 of ATPH exemplifies the general rules by which PPR proteins bind specific nucleotides: firstly, a residue at the 6 position of one repeat (Thr178 in the previous example) forms a hydrogen bond with the base. The identity of this residue determines whether the repeat will bind a purine (adenine and guanine) or pyrimidine (cytosine and uracil). It appears that serine and threonine at position 6 are specific for purines, and asparagine at position 6 is specific for pyrimidines.[4] Secondly, a residue at position 1 of the next repeat (Val210 in the previous example) completes the specificity of the interaction. Through van der Waals interactions, this residue determines between A/G and C/U. Other amino acids further contribute to this mechanism, but the previously described rules always apply when PPR proteins bind RNA sequences with modularity.[5] This image shows the general code by which PPR proteins recognize and bind RNA in a modular fashion. RNA StabilizationPPR10 stabilizes RNA upstream and downstream of its binding sites by blocking exonucleases from degrading RNA in either the 5' or 3' direction.[2] Translation EnhancementThe mechanism by which PPR10 increases the rate of translation is still unknown. However, it is predicted that PPR10 binds to sequences near the ribosome binding site of the RNA transcript. By doing so, PPR10 prevents the ~20 nucleotides to which it is bound from base pairing to the ribosome binding site and impairing translation. Considering the bacterial origins of the chloroplast, it is interesting to note that the RNA stabilizing and translation enhancing properties of PPR10 in the platid mirror the some of the functions of small RNAs (smRNA) in bacteria. In bacterial cells, smRNAs similarly bind regions near ribosome binding sites of mRNA, preventing degradation by nucleases and increasing translation by preventing base-pairing to ribosome binding sites.[2]
Synthetic ApplicationsThere is potential. . . References
| ||||||||||||