Sandbox WWC9
From Proteopedia
Contents |
The µ-opioid Receptor (4DKL)
Introduction
Opioids are highly specific endogenous and exogenous ligands in function as a means of analgesia and bind to a family of receptor proteins known as the opioid receptors (ORs). The three types of opioid receptors (µ-, κ-, δ-, respectively) belong to the protein superfamily G-protein coupled receptors (GCPRs); transmembrane proteins consisting of 7 membrane-spanning alpha-helical structures in which the extracellular ligand binding pocket exists. The µ-OR, exists primarily in the brain, spinal cord, and in the periphery. (1)
|
| Amino Terminus | Carboxy Terminus |
Here we can see a for this protein. Hydrophobic portions of the protein appear in grey, whereas polar potions appear in pink. 'Hydrophobic'Polar
Structure
Architecture
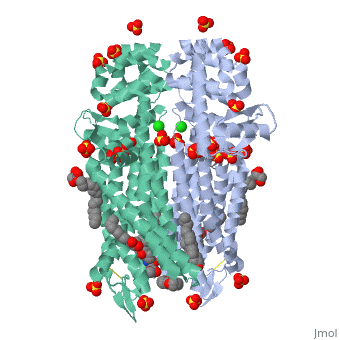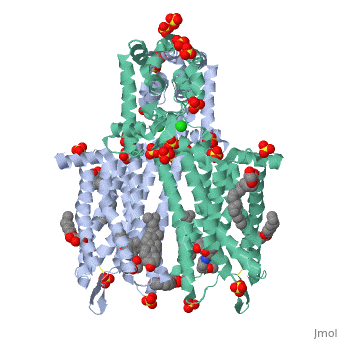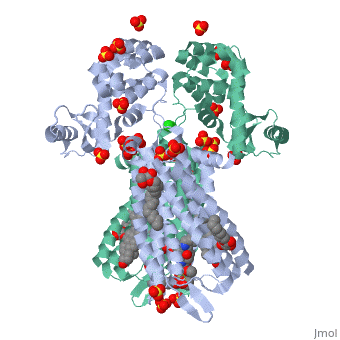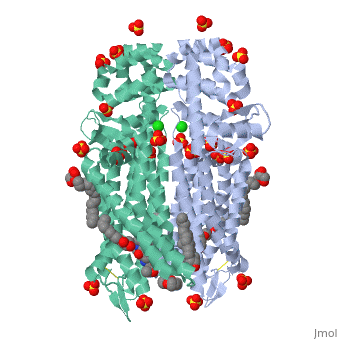
The µ-OR crystalized is that of the Mus musculus and has been given the protein database (PDB) identification 4DKL. This protein was crystalized on a 2.8 Å resolution with a bound irreversible morphinan antagonist β-funaltrexamine (β-FNA). The crystal structure of the protein shows the seven transmembrane helices characteristic of GPCRs that are connected by three extracellular loops and three intracellular loops. The receptors are arranges in dimers closely associated with transmembrane (TM) helices 5 and 6 (See image). The receptor also displays alternating aqueous and lipidic layers corresponding to transmembrane domains. (2) Binding site: The µ-OR, unlike most GPCRs that possess a buried binding site, contains an open solvent filled binding site of approximately 2173 Å3 and an accessible pocket area of 237Å2. (2,3)
References
1. Julian, R., Advokat, C., & Comaty, J. (2011). A Primer of Drug Action (Twelfth Edition). Worth Publishers. 2. Manglic, A.; Kruse , A. C.; Kobilka, T. S.; Thian, F. S.; Mathiesen, J. M.; Sunahara, R. K.; Pardo, L.; Weis, W. I.; Kobilka, B. K.; Granier, S. Crystal Structure Of the µ-Opioid Receptor Bound to a Morphinan Antagonist. Nature. 2012, 485, 321–326.
3. Cui, X.; Yeliseev, A.; Liu, R. Ligand Interaction, Binding Site And G Protein Activation of the Mu Opioid Receptor. Eur J Pharmacol. 2013, 702, 309–315.
4. Al-Hasani, R.; Bruchas, M. R. Molecular Mechanisms Of Opioid Receptor-Dependent Signaling and Behavior. Anesthesiology . 2011, 115, 1363–1381.