Function
DNA glycosylase (DG) are enzymes which remove damaged DNA bases by flipping them out of the double helix followed by their cleavage. Monofunctional DG have only glycosylase activity; bifunctional DG also act as lysates; ADG, UDG, TDG remove adenine, uracil, thymine from DNA.[1]
- Adenine DNA glycosylase is also called MutY. MutY has a role in prevention of DNA mutations resulting from oxidative damage forming the mutated base oxoG. DNA polymerase misreads oxoG and pairs it with adenine instead of cytosine. MutY removes adenine from the mismatched oxoG:A pair. MutY contains a 4Fe4S cluster.
- Thymine DNA glycosylase removes thymine moieties from mismatched G/T, C/T and T/T.
- Smug1 is a single-strand selective monofunctional UDG.
- 8-oxoguanine DNA glycosylase recognize oxidized bases.
- Human 8-oxoguanine GD is named hOgg1. See details on Ogg1 in 8-Oxoguanine Glycosylase.
- Methyladenine DNA glycosylase recognize methylated adenine; for details see Alka1.
- Formamidopyrimidine DG (FPG) or MutM or Fapy-DNA glycosylase removes oxidized purines from damaged DNA. More about FPG in
- Fpg Nei Protein Family
- Fpg Nei Protein Superfamily.
Structural highlights
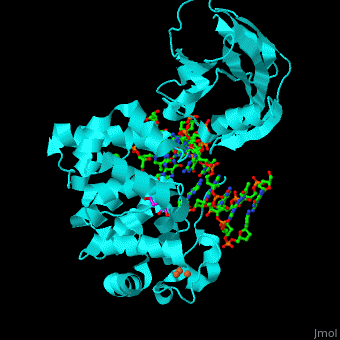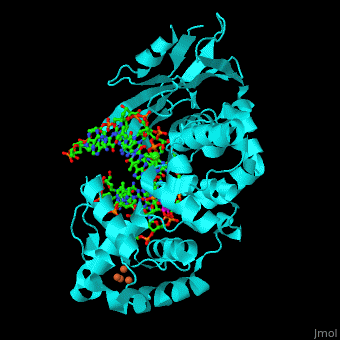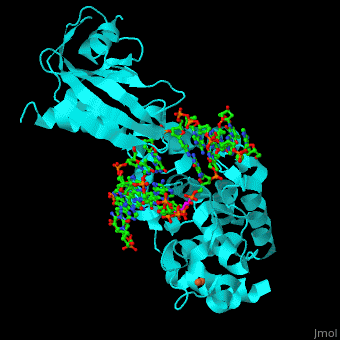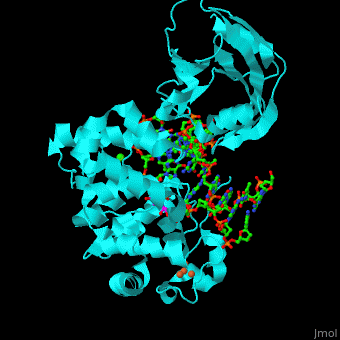
MutY uses base flipping to twist the mispaired adenine out of the DNA helix and into the MutY active site. MutY contains 2 domains: C-terminal domain and catalytic domain containing a helix-hairpin-helix.
- .
- .
- .
- (PDB code 1rrs)[2]. Water molecules shown as red spheres.