This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Instructions
Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [3] or to the article describing Jmol [4] to the rescue.
Biological Function
Structural Overview
Structural highlights
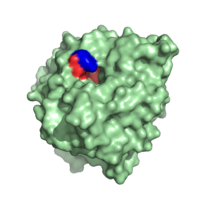
Carboxypeptidase A in
B. taurus. The red highlights the hydrophobic binding pocket while the blue highlights Y248.
To the left is the of Carboxypeptidase A in B. taurus, one of the molecules most important structural features. This binding pocket is the site at which C-terminal side chain of the substrate binds. It is formed by amino acid residues I243, I247, A250, G252, G253, S254 and I255.
Nearby, there is also Y198 which serves as the recognition site for the sidechain next to the C-terminal residue.
Mechanism of Action
Zinc Ligand(s)
Other Ligands
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.

