Sandbox Reserved 1071
From Proteopedia
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
|
Contents |
DgcZ from E. coli
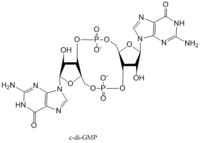
Diguanylate cyclases synthesize cyclic dimeric-GMP (c-di-GMP) from two GTP molecules. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc) , a polysaccharide required for E. coli biofilm production. This biofilm allows E. coli to adhere to extracellular surfaces. The DgcZ protein is made of two domains: the catalytic GGEEF domain responsible for sythnesizing c-di-GMP and the regulatory CZB domain that binds zinc. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function. DgcZ binds zinc with sub-femtomolar affinity, making it very likely that zinc will bind in the CZB domain.
Structure
E. coli DgcZ is a protein made of two domains each of which is a symmetric homodimer. The GGEEF domain is catalytic in that it contains the active sites used for cyclizing GTP into c-di-GMP. The CZB domain is used for ligand-mediated regulation of c-di-GMP production. Zinc binds as an allosteric inhibitor in coordination with four residues to shift the protein into an inactive conformation.
Catalytic GGEEF Domain
The GGEEF domain is made of a central five-stranded β-sheet with five α-helices surrounding it. Each dimer contains an active half-site that, when combined together in a productive conformation, form the entire active site. Each half-site binds one GTP. The guanyl base forms hydrogen bonds with Asp-173 and Asn-182 to hold it in the active site. A Mg2+ ion stabilizes the negative charges on the phosphate groups. When in the productive conformation, each GTP is held in close proximity with the α-phosphate groups overlapping C3 of the ribose. This conformation allows the α-phospate of one GTP to react with alcohol on C3 on the ribose of the other GTP, resulting in a cyclization of the two molecules into c-di-GMP.
CZB Domain
The CZB domain is responsible for regulating the function of DgcZ. It is composed of two dimers, each with four antiparallel α-helices. Zinc forms a tetrahedral coordination to the H22 residue of α1, Cys52 of α2, and His79 and His83 of α3. These four residues bind zinc with a very high affinity even at 10-16M concentrations. When not coordinated to zinc, the CZB domain adopts a conformation that shifts hydrophobic residues on the α-helices into the center and shifts the GGEEF domain into its productive conformation. Zahringer et al. mutated Cys52 to Ala, resulting in a lack of coordination on α2. Zinc still coordinates to the three His residues with the Cys52Ala mutation, but α2 is free to move and expose the Zinc binding pocket. This exposure was found to lower the protein's affinity for zinc.