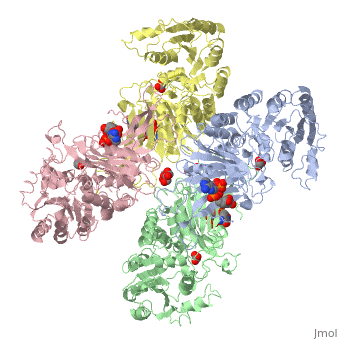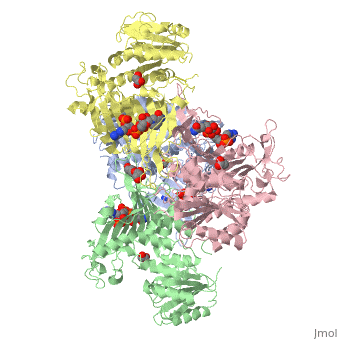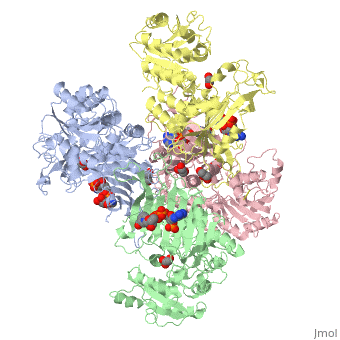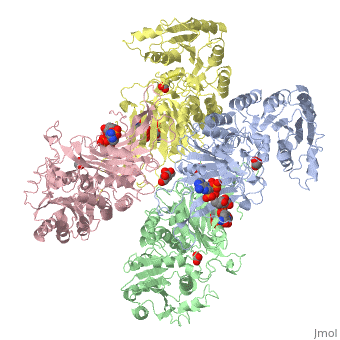
Overall, the structure of Glucose-6-Phosphate Dehydrogenase (G6PD) is a tetramer, composed of a dimer of dimers. Glucose-6-Phosphate Dehydrogenase also features a structural nicotinamide adenine, which is only present in eukaryotes and is rare biologically. Glucose-6-Phosphate Dehydrogenase is 515 amino acids long.
Function
Glucose-6-Phosphate Dehydrogenase is the rate-limiting enzyme in the pentose phosphate pathway. GGPD converts Glucose-6-Phosphate into 6-phosphoglucono-δ-lactone. Dysfunction of G6PD has multiple down stream effects. The pentose phosphate pathway synthesizes precursors for multiple other biosynthetic pathways. For example, it synthesizes nucleotide precursors for DNA synthesis.
Disease
Glucose-6-Phosphate Dehydrogenase deficiency is associated with many diseases, some of which include Hemolytic Anemia and Favism. Hemolytic Anemia results in erythrocytes being destroyed before their typical life span is over. Hemolytic anemia can lead to many problems, including cardiovascular arrhythmia, cardiomegaly (enlarged heart), and fatigue. Hemolytic anemia can present with different severity, but can be treated with drugs.
Favism is a disease associated with G6PD deficiency that can cause oxidative stress as a result of consuming fava beans. Favism and G6PD are X-linked diseases that are thus more prevalent in men than women.
Relevance
Glucose-6-Phosphate Dehydrogenase plays a key role in maintaining homeostasis. Not only does it catalyze the rate limiting step of the oxidative pentose phosphate pathway, it maintains the shape of red blood cells by protecting against potential deadly reactive oxygen species. Glucose-6-Phosphate dehydrogenase deficiency is the most common enzyme deficiency in humans, affecting over 400 million people worldwide. G6PD deficiency resulted in 4100 deaths worldwide in 2013.
Structural highlights
The two dimers are held together in a tetramer by charge-charge interactions.