The protein we are focusing one is a protein kinase receptor to a family of ligands called angiopoietins. This receptor is a Tyrosine Kinase TIE2. We are going to analyze the kinase domain of this protein
It acts as cell-surface receptor for the ligands ANGPT1, ANGPT2 and ANGPT4 and regulates among others angiogenesis, endothelial cell survival and maintenance of vascular quiescence. It is important in the regulation of both normal physiologic and pathologic angiogenesis. The later is a fundamental step in the transition of tumors from a benign state to a malignant one.
Angiogenesis is the process in which new blood vessels are formed from pre-existing blood vessels. The growth of these new blood vessels requires migration and proliferation of endothelial cells (ECs). It is an event controlled by angiogenic growth factors such as vascular endothelial growth factor (VEGF).
While ANGPT1 is a TIE2 agonist and has a higher binding affinity to it than ANGPT2, ANGPT2 can act as a context-dependent agonist. Thus, the ANGPT/TIE2 kinase signaling pathway is an attractive anti-vascular target.
Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
In vascular development, cellular processes are controlled by molecular signal transduction pathways. Those are under the influence of growth factor receptors : the tyrosine kinases present on the surface of endothelial cells. When ligands bind to these receptors, in this case ANGPT1 or ANGPT2, there is oligomerization provoking the activation of the kinase and autophosphorylation.
• Ligand and their binding
TIE2 maintains the vascular integrity of mature vessels by enhancing endothelial barrier function and inhibiting apoptosis of endothelial cells.
ANGPT1 is a TIE2 agonist : in vitro, it binds to TIE2 and induces its activation via tyrosine phosphorylation. In vivo, it was proven that inactivation of ANGPT1 or over expression of ANGPT2 produce similar effects.
ANGPT2 is a competitive antagonist of TIE2 or a partial agonist of TIE2 depending on the context. In stressed ECs, one recent report suggests that ANGPT2 may activate TIE2 signaling in the absence of ANGPT1 and in high concentrations.
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
Disease
• Dominantly inherited venous malformations (VMCM)
Disease description: A vascular morphogenesis error characterized by dilated serpiginous channels. All mutations occur in the protein kinase domain.
– Mutation in position 849 : Arginine → Tryptophane: Change from large size and basic (R) to large size and aromatic (W). Increased autophosphorylation and kinase activation; no effect on location at membrane.
Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation.
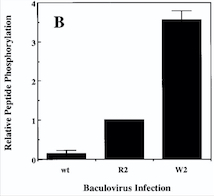
Diagram : Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors
(B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine → Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells.
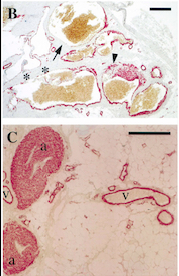
With this mutation, Venous Malformations (VMs) contain a Disproportionately high ratio of Endothelial Cells (ECs) to Smooth Muscle Cells (SMCs)
Pictures of immunohistochemistry of VMs with Antibodies against Smooth Muscle Cells 𝛂-Actin
B = Abnormal channels
C = Normal veins (v) and arteries (a)
Scale bars, 200 𝛍m.
Antibodies directed against SMCs 𝛂-Actin from cells with VMs show that the vessels have a specific and abnormal staining (B) compared to normal vessels (C)
Relevance


