Relevance
While the function of cytochrome b2 is to couple L-lactate dehydrogenation to cytochrome c reduction; the mutant ARG289LYS is changing the kinetics of the reactions. It is rising the Ki of several components in comparison to the wild-type, while kcat and KM are also changed by a factor of 10. It changes also the induction by L-lactate.
R289K-b(2)
eing a component of the mitochondrial inter membrane space
Structural highlights
Global Symmetry Cyclic - C4
Global Stoichiometry Homotetramer A4
Flavocytochrome b(2) is a tetrameric enzyme [Jacq and Lederer, 1972]. Each of the four identical subunits is composed by one single polypeptide chain.
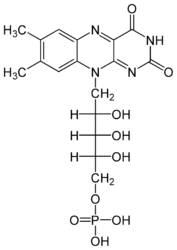
Each subunit contains a binding site for the selectively non-covalently binding of the cofactor FM3- (FlavinMonoNucleotide),
multiple image
| [[right
|center|border|180x180px|Image:Flav.png]] | Image:Flav.png |
| [[Flavinmononucleotide
|center|border|180x180px|Image:Hfdbtg.JPG]] | Image:Hfdbtg.JPG |
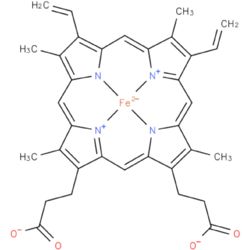
| [[heme b(2) cofactor
|center|border|180x180px|]] | |
|
https://www.wikiwand.com/de/Flavinmononukleotid
as well as one in with the iron complexed in the tetrapyrrole ring interacts with heme b(2-) cofactor [Risler and Groudinsky, 1973].
https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:60344
The amino acid sequence in the heme binding region was first determined by Guidard et al. (1974).
For every subunit the crystallized preparation analysis determined a molecular weight of the chain of 36 kD [Appleby and Morton] and the chain of 21 kD [Jacq and Lederer, 1974].
Arginin 289 to Lysine
http://www.worthington-biochem.com/yldhs/