Copper is one of the most important metal involed in multiple enzyme catalysed reactions as a cofactor. In living organisms its function is related to the redox property of the copper. However it is toxic at all co,ce,tration, from the lower to the higher, and it need to be strictly controled in living organisms by molecular mechanisms.[1]
Function
Multicopper oxidases are enzymes involved in copper homeostasis. Copper as many metal ions is used in multiple biological processes such as detoxification of oxygen free radicals and pigmentation. However copper present in a cell bot bounded to a protein if harmfull and can cause cellular damage. It need to be regulated.
Multicopper oxidase might also be involved in the regulation of metal transport.
Multicopper oxidases are abble to oxiise their substrate. They accept an electron in the and transfer it to the trinuclear copper centre. The dioxygen bind to the trinuclear center and recieve four electron. It is transformed into two molecules of water.[2] Three copper centres exist that can be differentiate spectroscopically: Type 1 or blue (), type 2 or normal (Cu1004) and type 3 or coupled binuclear (1002 and 1003).[3][4]
Disease
If the amino acids 500 and 501 are mutated from CH to SR, the residual activity and loss of resistance to copper. E. Coli die.
Relevance
Structural highlights
Multicopper Oxidase CueO 4e9s is a protein containing one chain with sequence from Ecoli. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance.
|
| Ligands: | 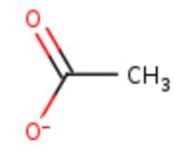 Figure 1: Acetate ion. [5] |
| Related: | 4e9p, 4e9q, 4e9r, 4e9t |
| Gene: | cueO, yacK, b0123, JW0119 (ECOLI) |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT |
In the chain, 5 ligands are present: an acetate ion and 4 copper ions.
In the chain, 5 ligands are present: an acetate ion and 4 copper ions.
|
| Cu1001: The copper ion is bound thanks to 3 metal protein interactions with H443, H505 and C500, structure stabilised by L502, M510 by hydrogen bonds |
| Cu1002: 2 atoms of Cu, bonds twice by metal interaction (2x3) with 3 histidine : H501, H103, H141 stabilised by hydrophobic contact with W139 |
| Cu1003: 2 CU bond with 3histidine : H499, H143, H448 by 2 metal interactions. h448 is stabilised by Pi interactions with [CU]1004 and H101 |
| Cu1004: one single atome of CU bonds once by metal interactions with 2 H ( and one ACT[1005]) : H101 and H446 stabilised by Pi interactions with H103 and H448. [CU]1004 has Pi interactions with H103 and H448. H113 and H446 Pi interactions |
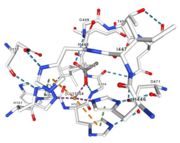 Figure 6: Acetate ion 1005 [10] | ACT[1005]: is linked by hydrogen bonds to G104 and G449 |
Cu1002, Cu1003, Cu1004 and ACT[1005] are near in the space, the 3 CU form a triangle.
The amino acid sequence is:
AERPTLPIPDLLTTDARNRIQLTIGAGQSTFGGKTATTWGYNGNLLGPAVKLQRGKAVTVDIYNQLTEETTLHW
HGLEVPGEVDGGPQGIIPPGGKRSVTLNVDQPAATCWFHPHQHGKTGRQVAMGLAGLVVIEDDEILKLMLPKQWGIDDVPVIVQDKKFSADGQIDYQLDVMTAAVGWFGDTLLTNGAIYPQHAAPRGWLRLRLLNGCNARSLNFATSDNRPLYVIASDGGLLPEPVKVSELPVLMGERFEVLVEVNDNKPFDLVTLPVSQMGMAIAPFDKPHPVMRIQPIAISASGALPDTLSSLPALPSLEGLTVRKLQLSMDPMLDMMGMQMLMEKYGDQAMAGMDHSQMMGHMGHGNMNHMNHGGKFDFHHANKINGQAFDMNKPMFAAAKGQYERWVISGVGDMMLHPFHIHGTQFRILSENGKPPAAHRAGWKDTVKVEGNVSEVLVKFNHDAPKEHAYMAHCHLLEHEDTGMMLGFTVG
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.