Sandbox TYRP1
From Proteopedia
Contents |
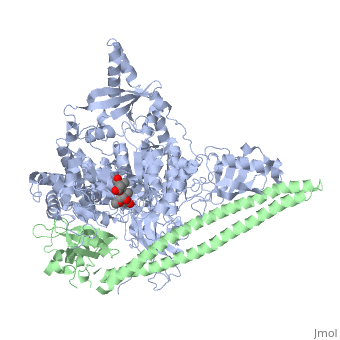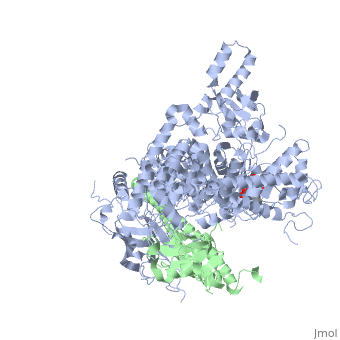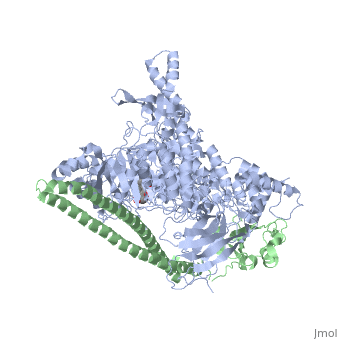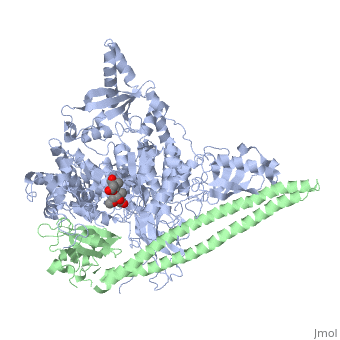
Tyrosinase related protein 1 (5M8L)
|
Human Tyrosinase related protein 1 (TYRP1) is a Cu2+/Zn2+ metalloenzyme found in Humans. It is expressed in melanocytes where it plays an important role in pigmentation. TYRP1 is also involved in melanoma and albinism. Therefore, it represents an interesting target for therapy [1]. TYRP1 can also be called : 5,6-dihydroxyindole-2-carboxylic acid oxidase (DHICA oxidase); Catalase B or Glycoprotein 75 (gp75).
Synthesis and transport
Human Tyrosinase related protein 1 is encoded by the TYRP1 gene, which is located on the chromosome 9p23. The protein is expressed in melanosomes and on the surface of melanocytes and melanoma cells [2]. TYRP1 protein is synthesized in the nucleus of melanosomes thanks to a signaling sequence. Then, it will be translated by the ribosomesand the protein will be directly transported in the endoplasmic reticulum. Then it will be transported through the Golgi to a specific organelles called melanosomes, where pigments are synthesized [3]. During its maturation, TYRP1 is glycosylated in asparagine in positions 96; 104; 181; 304; 350 and 395. The sorting in the trans-Golgi and transport of the TYRP1 protein to melanosome is dependant of several proteins such as theThis page, as it appeared on November 15, 2010, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
Additional Resources
- See: Cancer For Additional Proteins involved in the disease.
- See: Oncogenes for Additional examples of oncogenes and tumor suppressor genes.
References
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Liu TF, Kandala G, Setaluri V. PDZ domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase-related protein-1). J Biol Chem. 2001 Sep 21;276(38):35768-77. doi: 10.1074/jbc.M103585200. Epub 2001, Jul 5. PMID:11441007 doi:http://dx.doi.org/10.1074/jbc.M103585200
- ↑ Liu TF, Kandala G, Setaluri V. PDZ domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase-related protein-1). J Biol Chem. 2001 Sep 21;276(38):35768-77. doi: 10.1074/jbc.M103585200. Epub 2001, Jul 5. PMID:11441007 doi:http://dx.doi.org/10.1074/jbc.M103585200
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Kobayashi T, Urabe K, Winder A, Jimenez-Cervantes C, Imokawa G, Brewington T, Solano F, Garcia-Borron JC, Hearing VJ. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994 Dec 15;13(24):5818-25. PMID:7813420
- ↑ Liu TF, Kandala G, Setaluri V. PDZ domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase-related protein-1). J Biol Chem. 2001 Sep 21;276(38):35768-77. doi: 10.1074/jbc.M103585200. Epub 2001, Jul 5. PMID:11441007 doi:http://dx.doi.org/10.1074/jbc.M103585200
- ↑ Lai X, Wichers HJ, Soler-Lopez M, Dijkstra BW. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew Chem Int Ed Engl. 2017 Aug 7;56(33):9812-9815. doi: 10.1002/anie.201704616., Epub 2017 Jul 17. PMID:28661582 doi:http://dx.doi.org/10.1002/anie.201704616
- ↑ doi: https://dx.doi.org/10.1007/s003359900678
- ↑ Vijayasaradhi S, Bouchard B, Houghton AN. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med. 1990 Apr 1;171(4):1375-80. doi: 10.1084/jem.171.4.1375. PMID:2324688 doi:http://dx.doi.org/10.1084/jem.171.4.1375
- ↑ Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011 Apr;5(2):150-5. doi: 10.1016/j.molonc.2011.01.006. Epub 2011 Feb , 3. PMID:21324755 doi:http://dx.doi.org/10.1016/j.molonc.2011.01.006
- ↑ Journe F, Id Boufker H, Van Kempen L, Galibert MD, Wiedig M, Sales F, Theunis A, Nonclercq D, Frau A, Laurent G, Awada A, Ghanem G. TYRP1 mRNA expression in melanoma metastases correlates with clinical outcome. Br J Cancer. 2011 Nov 22;105(11):1726-32. doi: 10.1038/bjc.2011.451. Epub 2011, Nov 1. PMID:22045183 doi:http://dx.doi.org/10.1038/bjc.2011.451
- ↑ 16.0 16.1 16.2 16.3 16.4 [Xuelei Lai, Harry J. Wichers, Montserrat Soler‐Lopez, Bauke W. Dijkstra. Structure and Function of Human Tyrosinase and Tyrosinase‐Related Proteins. 2018 Jan 2 Epub 2017 Nov 28 PMID: 29052256 https://www.ncbi.nlm.nih.gov/pubmed/29052256 DOI: 10.1002/chem.201704410 https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201704410]
- ↑ 17.0 17.1 17.2 17.3 17.4 [Decker. H, Tuczek.F. The Recent Crystal Structure of Human Tyrosinase Related Protein 1 (HsTYRP1) Solves an Old Problem and Poses a New One. 2017 Nov 13. Epub 2017 Oct 9 PMID: 28990327 https://www.ncbi.nlm.nih.gov/pubmed/28990327 DOI: 10.1002/anie.201708214 https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201708214]
- ↑ Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002 Aug;27(8):426-32. PMID:12151228
- ↑ 19.0 19.1 19.2 Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kgamma. Nature. 2000 Aug 24;406(6798):897-902. PMID:10972292 doi:10.1038/35022585
- ↑ Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999 Apr 16;274(16):10963-8. PMID:10196176
- ↑ Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007 Jul 13;317(5835):239-42. PMID:17626883 doi:317/5835/239
- ↑ Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33-9. PMID:1851250 doi:http://dx.doi.org/10.1038/351033a0
- ↑ Hoedemaeker FJ, Siegal G, Roe SM, Driscoll PC, Abrahams JP. Crystal structure of the C-terminal SH2 domain of the p85alpha regulatory subunit of phosphoinositide 3-kinase: an SH2 domain mimicking its own substrate. J Mol Biol. 1999 Oct 1;292(4):763-70. PMID:10525402 doi:http://dx.doi.org/10.1006/jmbi.1999.3111
- ↑ Harris SJ, Foster JG, Ward SG. PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr Opin Investig Drugs. 2009 Nov;10(11):1151-62. PMID:19876783
[4], the membrane associated transporter protein (MATP) [5] and the GAIP interacting protein (GIPC) [6]. The final TYRP1 protein is 537 amino-acids long. TYRP1 is transported to the membrane by the biogenesis of lysosome-related organelles complex 1 (BLOC-1) (wikipedia). The amino-terminal domain will be oriented in the lumen of the melanosome, and the carboxy terminal domain in the cytoplasm of the melanocyte [7]. TYRP1 is found only in the membrane of mature stage III and IV melanosomes [8].
Function
Role in melanocytes First, TYRP1 has a role in melanin biosynthesis. Indeed, this enzyme has a catalytic function in the melanin biosynthetic pathway. In mouse, when a Cu2+ cation is bound, the protein catalyzes the oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) into indole-5,6-quinone-2-carboxylic acid. This protein is also able to catalyze the oxidation of 5,6-dihydroxyindole (DHI) into indole-5,6-quinone. Both products will allow to obtain eu-melanin, while pheo-melanin is obtain thanks to TYRP2 activity [9]. The activity of the TYRP1 enzyme increase when the serine residues in position 505 and 509 are phosphorylated [10]. However, this mechanism does not happens in Humans because Human TYRP1 does not have the DHCIA activity. This can be explain by the fact that the nature of ions in the active site is different. Indeed, a Zn2+ ion bounds the active site of the TYRP1 enzyme instead of a Cu2+, which is responsible for a different activity. To conclude, the exact role of TYRP1 in pigmentation remains still unclear [11]. Moreover, no gene polymorphism has been observed among caucasian population, despite the variation of hair and skin colors [12]. In addition, the mouse homolog of the TYRP1 is involved in melanocytes differenciation too. Therefore, it could be used as a differentiation marker [13]. In humans, the exact role of TYRP1 in differentiation of melanocyte is unclear. However, it is supposed that the protein is involved in the mechanism, as it is involved in pigmentation.
Role in melanoma TYRP1 also have a role in progression of melanoma. In fact, as TYRP1 is involved in the proliferation and differentiation of melanocytes, a mutation of the protein is associated with a higher risk for melanoma [14]. Therefore, the level of expression of TYRP1 mRNA is prognostic marker [15].
Structural highlights
Main domains and lattices
TYRP1 is a globular monomeric protein. It is composed of several domains: a short peptide signal on the N-terminal side followed by a large intra-melanosomal domain. This intra-melanosomal domain contain a rich-cysteine domain and a catalytic tyrosinase-like subdomain with two ion-binding sites.[16] The next part of the sequence is composed of a transmembrane alpha helix followed by a short cytoplasmic sequence on the C-terminal chain. [17]
The cystein-rich domain and the tyrosinase-like subdomain stongly interact together by the last loop of the cystein-rich domain preceding the N-terminal domain. The role of the cystein-rich domain is still unknown, it is only found in mammalians but 3D-structure highlights two pairs of short antiparallel beta-strands which create loops. This domain is stabilize by five disulfide bounds and is located at the opposite of the active site. It is sad that the cystein_rich domain might help to the formation of a complexe between TYR and TYRP2.[16]
The active site
The active site is delimited by four helice and contain the binuclearmetal binding site. (image à faire)
Comparison between enzymes of Tyrosinase family
In mammals, three enzymes of Tyrosinase family may be involved in biosynthesis of melanin. Tyrosinase (TYR) reacts two times in the mechanism whereas Tyrosinase Related Protein 1 and 2 (TYRP1 and TYRP2) probably catalyze only one reaction in this biosynthesis. TYR is an oxydoreductase,TYRP2 seems to act as a tautomerase and the exact role in melanin synthesis of human TYRP1 is still under debate. In fact in mices, TYRP1 can especially catalyze the reaction of DHICA in eumelanin but human TYR can also do the same. It is said that TYRP1 can play a significant role in proliferation of melanosomes.[17]
(Image à faire reaction chimique)
Similarities:
All three melanogenic enzymes are metal-containing glycoproteins and have a single transmembrane alpha-helix. 40% of their amino acid sequence is exactly the same and 70% of their sequences are analogous [16] In fact, multiple human sequence aligment show that Tyrosinase (TYR), TYRP1 and 2 have following similar domains. First, a short peptide signal on the N-terminal side followed by a large intra-melanosomal domain. This intra-melanosomal domain contain a rich-cysteine domain and a catalytic tyrosinase-like subdomain with two ion-binding sites.[16] After that there is a transmembrane alpha helix followed by a short cytoplasmic sequence on the C-terminal chain. [17]
(image à faire)
These three proteins share similar active sites. Metal ions interact with three histidines.
Differences:
The main difference between these three enzymes is the nature of metal ions they bind on the active site. TYRP1 and TYRP2 bind two zinc ions whereas TYR binds two copper ions
Interet des ions !!! The binds between the protein and its inhibitors are not affected by change in hydrogen bounds. It can be interesting to study this property to design better inhibitors. The future discovery of TRP1 role in melanin synthesis may be a breakthrough for cosmetic industry. [17]
References
[16] Xuelei Lai, Harry J. Wichers, Montserrat Soler‐Lopez, Bauke W. Dijkstra. Structure and Function of Human Tyrosinase and Tyrosinase‐Related Proteins. 2018 Jan 2 Epub 2017 Nov 28 PMID: 29052256 https://www.ncbi.nlm.nih.gov/pubmed/29052256 DOI: 10.1002/chem.201704410 https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201704410
[17] Decker. H, Tuczek.F. The Recent Crystal Structure of Human Tyrosinase Related Protein 1 (HsTYRP1) Solves an Old Problem and Poses a New One. 2017 Nov 13. Epub 2017 Oct 9 PMID: 28990327 https://www.ncbi.nlm.nih.gov/pubmed/28990327 DOI: 10.1002/anie.201708214 https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201708214