User:Sunghwan Cho/Sandbox reserved 899
From Proteopedia
|
Contents |
Introduction to Cytochrome P450 and CYP2C9*30
Cytochrome P450(CYP) is a monooxygenase and heme-containing enzyme found in the endoplasmic reticulum(ER) membrane. CYPs have the function of the biogenesis of sterols and hormones. Also, it involves in the detoxification of foreign chemicals and the metabolism of drugs. The alphabet "P" stands for the pigment from liver microsomes, and the wavelength at 450nm is measured when heme iron in CYP is reduced and interacts with carbon monoxide [1]. The major function of CYPs is to interact with a clinically available drug, including nonsteroidal anti-inflammatory drug (NASID) ibuprofen, antihypertensive losartan, and the anticoagulant warfarin; especially CYP3A4, CYP2D6, and CYP2C9 are enzymes that involve phase Ⅰ metabolism. CYPs have different family, sub-family, and the individual members of sub-family that provide a different function. For example, CYP2C9 is 2 for family, C for sub-family, and 9 for individual members of sub-family.
Furthermore, Single nucleotide polymorphism(SNP) renders more diversification rather than wild type classification. SNP is a variation in a single base pair in a DNA sequence that may lead to the variation in the amino acid of the protein. Such variations may influence the function of CYPs that leads to a negative effect on an individual's response, such as drug-drug interactions. [2], [3]. The general schematic representation of the CYP represented in Figure 1.
The Metabolism of Cytochrome P450
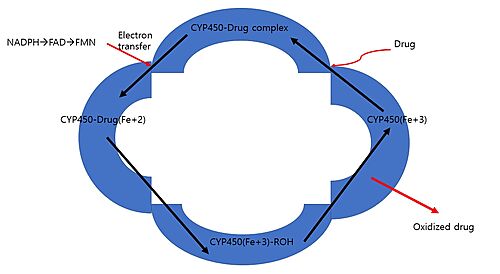
As cytochrome P450 reside on the ER membrane, to interact with a substrate, the substrate should be near in proximity to the enzyme.
Comparative analysis between CYP2C9 Wild type and CYP2C9*30
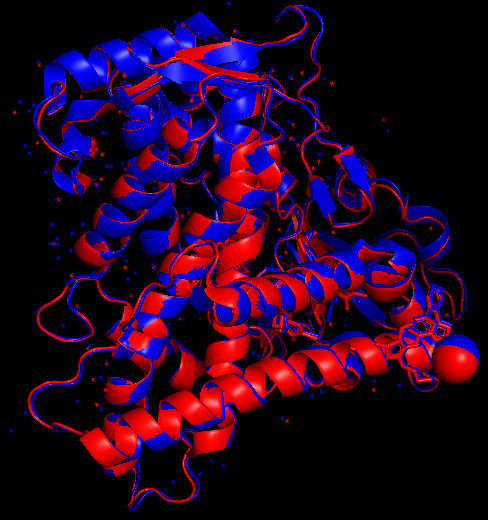
The structural feature of cytochrome P450 is a tertiary structure that is composed of a mixture of α-helix and β-sheet connected by loops.
Amino acid sequential difference
Structural highlights
Image:Star30 with losartan.pse This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ OMURA T, SATO R. A new cytochrome in liver microsomes. J Biol Chem. 1962 Apr;237:1375-6. PMID:14482007
- ↑ Meyer U.A., Zanger U., Skoda R., Grant D.M. (1989) Genetic Polymorphisms of Drug-Metabolizing Enzymes: Molecular Mechanisms. In: Dahl S.G., Gram L.F. (eds) Clinical Pharmacology in Psychiatry. Psychopharmacology Series, vol 7. Springer, Berlin, Heidelberg DOI https://doi.org/10.1007/978-3-642-74430-3_15
- ↑ Daly AK. Pharmacogenetics of the cytochromes P450. Curr Top Med Chem. 2004;4(16):1733-44. doi: 10.2174/1568026043387070. PMID:15579105 doi:http://dx.doi.org/10.2174/1568026043387070
- ↑ Brunton, L., Knollmann, B., & Hilal-Dandan, R. (2018). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York, N.Y.: McGraw-Hill Education LLC ISBN: 978-1-25-958473-2
- ↑ Ghosh, C., Hossain, M., Solanki, J., Dadas, A., Marchi, N., & Janigro, D. (2016). Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discovery Today, 21(10), 1609-1619. doi: 10.1016/j.drudis.2016.06.004
- ↑ Kessel, A., and Ben-Tal, N. (2018). Introduction to proteins_structure, function, and motion. 2nd ed.
