User:Wiatt Suich/Sandbox 1
From Proteopedia
Ectatomin is a 2 chain polypeptide protein, belongs to the ectatomin-ET subfamily.Ectataomin produces the toxic effects of the venom from the Ectatomma tuberculatum ant. The LD50 was determined to be 6.8 ug/kg when injected into mice and had a LD50 of 2.1ug/g when injected into cockroaches.[1]
|
Contents |
Process of Discovery
The Ectatomin protein structure was determined using two dimensional 1H NMR techniques. The MARDIGRAS program created accurate distance constraints for the protein to protein measurements. The DIANA program was used to find the disulfide bridges.The protein was ran through the CHARMm program for refinement and touchups.The rms deviation was determined to be .75 A for the heavy backbone atoms, for overall heavy atoms it was 1.25 A.[2]
Ectatomma tuberculatum ant
The Ectatomma tuberculatum ant that produces the ectatomin protein lives in the upper region of South America region and the lower North America region. This ant is one of the 15 species that make up the Ectatomma family. This ant normally builds nests on the ground around trees and are built up around three types of ant.There are workers, micro queens(mircogynes), and queens(macrogynes). The queen is three times larger than micro queens, though both queens can be inseminated. The queens are able to produce a larger amount of larva than micro queens.[3] The micro queens are also known as Ectatomma Parasiticum, and produce nearly identical larva as the queens but produce larva that are biased to the microqueen survival. When enough larva have been born, the microqueens and her workers will attempt to usurp the colonies of the Ectatomma tuberculatum.[4] The part of the body where the toxin originates is the venom gland. This gland is connected to a stinger which allows the ant to stab its prey and deliver the toxin. Though the main purpose of the toxin is to kill the prey or attacker,the venom can be used for colony asepsis to prevent infections in food and other ants.[3]
Structural highlights
is a simple toxin protein with a molecular weight of 7928 Da and a pl of 9.95.[5] The protein is made up of two polypeptide chains,that are homologous and amphiphilic. The A chain is 37 amino acids and the B chain is 34 amino acid in length. The two chains are held together by a .[6] The chains are made from antiparallel alpha helices, and are connected by a made up from a disulfide bridge and four amino acids. This hinge region creates additional stability by forming a hairpin unit and helps the disulfide bonds remain stable.The post translational modifications that occur on this protein are disulfide bonds. These bonds connect the 12-34 sites of the A subunit and also connect the 22 of subunit A to the 20 site of sub unit B. There is a final disulfide bond that connects the 10 site to the 32 site of subunit B. These are the bonds that hold the protein in the bundle shape that you see in the diagram.[1]
Function
Ectatomin is the protein that causes the toxic effect of the venom. Ecataomin targets the cell membrane, creating pores that cause ion leakage and low membrane resistance. The pores are formed due to the ectatomin channel forming ability. These channel/pores are formed selectively on the membrane and occur at positive cis-potential locations. At these locations, two ectatomin molecules must be present in order for the formation of a pore. The protein is able to insert itself into the membrane and interact with the protein kinases part of the signaling cascade. At a lower concentration ectatomin is able to enter the membrane but is not able to penetrate inside the cell. When raised to a higher concentration, the protein instead inhibits the autophosphorylation of pp60c-src protein tyrosine kinase.The pp60c-src protein has multiple purposes in cells. These activities include gene transcription, cell cycle progression, apoptosis, and cell adhesion. Higher concentrations are required for hemolytic and cytolytic effects to occur, the toxin will also act more like a detergent similar to mellitin. Ectatomin causes the inhibition of the calcium channels. The ectatomin protein was found to have a higher affinity for binding to the gated calcium channels after the channels had been stimulated by B-adrenergic.[5] The main effect that ectatomin has on calcium channels is the destruction of isoproterenol and forskolin sensitive components of the lCa channel. Isoproterenol is a agonist of beta adrenoreceptors, when bound to calcium channels the protein initially increases the rate of calcium ions but at longer durations the protein will inhibit the calcium channels, lowering the amount of ions released. Ectatomin targets this component to stop isoproterenol from binding to the channels and inhibiting the release of ions. This effect will have cells slowly leak ion from the pore channels formed by ectatomin and by their own calcium channels that now lack inhibitors. [7]
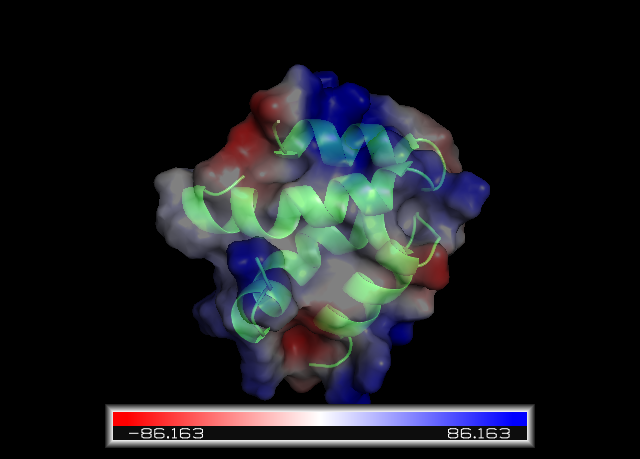
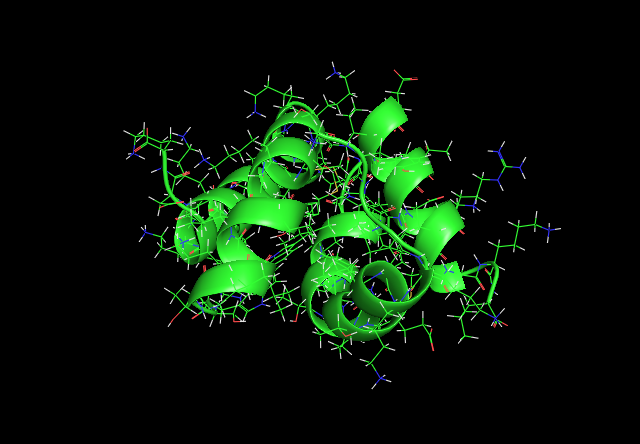
Pictures of Ectatomin
Ectatomin showing polar surface charges. Charge value shown on the bottom.
Ectatomin with all main and side chains showing.
References
- ↑ 1.0 1.1 “Ectatomin.” InterPro, ELIXIR Core Data Resource, www.ebi.ac.uk/interpro/entry/InterPro/IPR009458/.
- ↑ Nolde, D E, et al. “Three-Dimensional Structure of Ectatomin from Ectatomma Tuberculatum Ant Venom.” Journal of Biomolecular NMR, U.S. National Library of Medicine, Jan. 1995, www.ncbi.nlm.nih.gov/pubmed/7881269?dopt=Abstract
- ↑ 3.0 3.1 “Ectatomma Tuberculatum.” AntWiki, MediaWiki, www.antwiki.org/wiki/Ectatomma_tuberculatum.
- ↑ “Ectatomma Parasiticum.” AntWiki, MediaaWiki, www.antwiki.org/wiki/Ectatomma_parasiticum.
- ↑ 5.0 5.1 Pluzhnikov, Kirill, et al. “Analysis of Ectatomin Action on Cell Membranes.” FEBS Press, John Wiley & Sons, Ltd, 25 Dec. 2001, febs.onlinelibrary.wiley.com/doi/full/10.1046/j.1432-1327.1999.00426.x.
- ↑ Pluzhinikov, K A, et al. “Structure-Activity Study of the Basic Toxic Component of Venom from the Ant Ectatomma Tuberculatum.” Bioorganicheskaia Khimiia, U.S. National Library of Medicine, 1994, www.ncbi.nlm.nih.gov/pubmed/7826413?dopt=Abstract.
- ↑ Xiong, Z, et al. “Isoproterenol Modulates the Calcium Channels through Two Different Mechanisms in Smooth-Muscle Cells from Rabbit Portal Vein.” Pflugers Archiv : European Journal of Physiology, U.S. National Library of Medicine, Sept. 1994, www.ncbi.nlm.nih.gov/pubmed/7971166.