Function
[PCNA_HUMAN] Proliferating Cell Nuclear Antigen (PCNA) is a protein that acts as a DNA sliding clamp. It forms a homotrimer encircling the DNA and binds other peptides means known as PCNA interacting proteins. It acts as a processivity factor for DNA polymerases and other enzymes which act upon DNA. Examples of such are DNA polymerase (Dpo) δ in eukaryotic cells[1]. The increases in processivity are very pronounced. The number of basepairs processed before complex dissociation occurs is increased more than a thousandfold (~10bp[2] to ~80kbp[3]) and the speed of nucleotide incorporation rises about a hundredfold [4].
Relevance
PCNA is featured in many cellular pathways involving DNA. FEN1 bound to PCNA acts as a flap endonuclease and cleaves a displaced ssDNA (flap) containing oxidatively damaged dideoxyribose residue [5]. As stated above PCNA is also vital to formation of the procesive complex for DNA replication [6] and is featured even in gene expression and transcription when bound to GADD45A [7]. Thus PCNA is relevant in research and even medicine.
PCNA is useful in the diagnosis of high-grade dysplasia[8].
Structural highlights
p15 regulates DNA replication and repair by binding to PCNA. PCNA-p15 peptide complex shows the and has [9]. Of interest is the binding site contained at each face of the PCNA ring. Said site is formed by a C-terminal domain formed groove and an interdomain connecting loop (IDCL). Some partners do bind to the N-terminal domain as well. There are multiple PCNA interacting protein binding motifs, but the most common one is QxxΨxx∇∇ (where Ψ is a hydrophobic residue and ∇ is either the aromatic residue F or Y) [10][11]
Known mutations
S228I
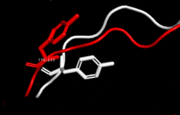
hPCNA-S228I disorder tyr133 difference
A disease causing variant in human, which causes a large conformational change of the PCNA interacting protein binding pocket. While the overal structure of the S228I mutant is mostly similar to the wild type, the change causes displacements extending from the mutation site, which affect the IDCL. Notably Tyr133, a highly conservated residue, rotates outwards by nearly 90° to prevent spherical conflicts with Ile228. The changes cause the binding cavity of S228I to be about a third of the size of its wild type equivalent and makes it incompetent for PCNA interacting protein binding. Such changes, if they were permanent, would however be lethal, as the removal of PCNA-FEN1 interactions is lethal on its own [12]. While some client proteins (e.q. [[p21CIP1]]) are left relatively unaffected even in this state, others' binding energetics are hugely disrupted (e.q. RNase H2B or FEN1). The explaination for survival of an individual carrying this mutation is sufficient malleability of IDCL (and thus of PCNA itself). Thus a PCNA interacting protein might be able to initiate an "induced-fit". Even here the mutant is disadvantaged as the wild type has B-factors ~40% higher than the mutant. Thus the mutation negatively affects even the IDCL dynamics. [13]. .
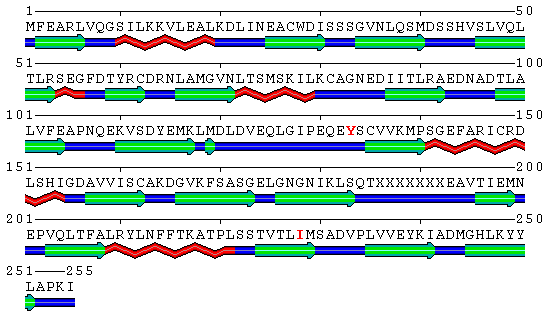
Human PCNA S228I mutant secondary structure with marked mutation and rotated Tyr133 marked red in sequence. Helices are marked red, sheets green and loops blue. Note the IDCL region (residues around 125).
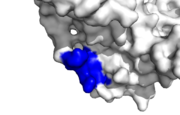
Human PCNA interacting protein healthy binding site, IDCL marked blue
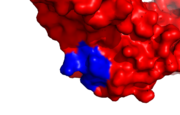
hPCNA-S228I PCNA interacting protein binding site, IDCL marked blue
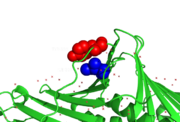
Tyr 133 is rotated by ~90° due to spherical conflict with Ile 228 in human PCNA mutant S228I.
Diseases
hPCNA-S228I disorder [14]
Symptoms resemble those of DNA damage and repair disorders (e.q. xenoderma pigmentosum). Among others heightened UV sensibility and deffects of nucleotide excission repair. DNA replication in patients seems to proceed close to the norm. While there have been observed similar disorder in yeast [15], their mechanism differs. The yeast diseases are caused by premature dissociation from DNA, while leaving PCNA interacting protein binding pocket unaffected. However there are two future avenues of research. The first is the aforementioned simillarity to xenoderma pigmentosum. XPG, a protein involved in the aforementioned disease, has a high level of sequence similarity in its PCNA binding motif to FEN1, a protein whose binding was shown to be disrupted by S228I. The second is the observed lack of interaction with RNase H2B thus intervening in the ribonucleotide excision repair pathway [16]
3D Structures of Proliferating Cell Nuclear Antigen
Proliferating cell nuclear antigen 3D structures





