Function
Ubiquitin-protein ligase (UPL) (E3) or E3 ubiquitin-protein ligase in combination with ubiquitin-conjugating enzyme (E2) causes the attachment of ubiquitin to lysine in a target protein[1]. UPL XIAP or X-linked Inhibitor of Apoptosis Protein stops apoptosis induced by viral infection or by overproduction of caspases. UPL XIAP binds caspase-3, -7 and -9[2]. UPL XIAP is inhibited by Smac – a death signaling protein.
For E3 ubiquitin-protein ligase parkin see Parkin.
Disease
XIAP mutations cause X-linked lymphoproliferative syndrome type 2[3]. Parkin UPL has a direct causal role in neurodegenerative disorders[4]. Cereblon nonsense mutation causes autosomal recessive nonsyndromic mental retardation[5].
Relevance
Overexpression of UPL XIAP functions as tumor marker. UPL cereblon is inhibited by thalidomide[6].
Structural highlights
UPL can contain several distinct domains like:
- WW domains which contain 2 tryptophans which bind proline-rich peptide;
- UBA – Ubiquitin-Associated Domain;
- TKB – Tyrosine Kinase Binding domain;
- C2 – an 8 β strand domain which coordinates targeting proteins toward cell membrane;
- Cereblon is a target for thalidomide;
- HECT – Homologous to the E6-AP C-terminus;
- RING – Really Intersting New Gene involved in binding to E2;
- FHA – Forkhead Associated domain recognizing phosphothreonine;
- IBR – involved in binding to E2;
- PHD finger – Plant Homeo Domain is 50-80 amino acid long which contains a CHC motif;
- SRA – SET and RING-Associated domain;
- TUDOR – a ca. 50 amino acids long and recognizes dimethylated arginine;
- CPH – protein-protein interaction domain;
- UBR – a ca. 70 amino acid long zinc finger-like domain;
- CUE – a ubiquitin-binding domain.
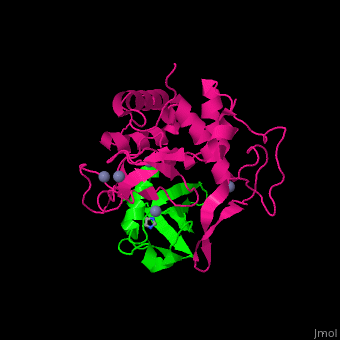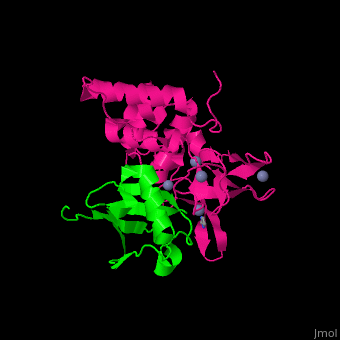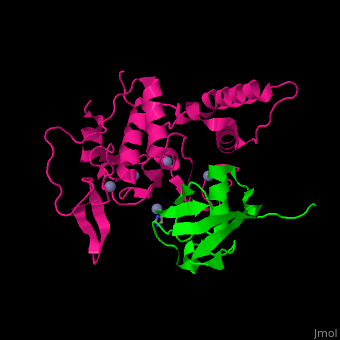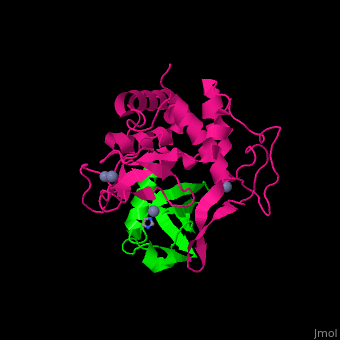
UPL XIAP consists of 3 BIR (baculoviral IAP repeat) domains, followed by UBA (ubiquitin-binding) domain and RING (C-terminal RING finger) domain. BIR2 inhibits caspase-3 and -7. BIR3 inhibits caspase-9.
UPL contains an thioester-forming cysteine which catalyzes the transfer of ubiquitin from E2 to a substrate lysine. The surface interacting residues of UPL and the acceptor ubiquitin are shown here[7].
For some details see
3D Structures of ubiquitin protein ligase
Ubiquitin protein ligase 3D structures