This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia or to the article describing Jmol [1] to the rescue.
Structure and Function
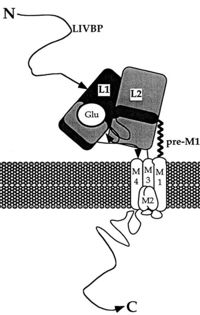
NR2A (GluN2A) is composed of Amino-terminal domain (ATD), segments S1 and S2 which formed ligand binding domain of glutamate, three transmembrane helices (M1, M3,and M4), cytoplasmic re-entrant pore loop (M2), and an intracellular C-terminal domain (CTD).
The ATD is constitued by first 383 aminoacids of NR2A. ATD is an alpha and beta protein class. Structure is bilobed and form clam-shell like structure which consists in two lobes linked by a flexible hinge region defining a central groove. [2] Zn2+ may insert between 2 lobes and induces closure of channel by changing conformation of ATD. Zn increase affinity of glutamate on the LBD which reminds the desensization of AMPA and Kainate receptor. [3]
ATD allow to modulate NMDA receptor. Difference between différents NR2 is mainly regulated by ATD because diversity of ATD can modulate traffic in endoplasmic reticulum ans then affect the localisation of NMDAr. ATD of NR2A increase glutamate affinity, control channel’s opening with high probability and open duration, control glutamate deactivationtime course.[4] [5]
LBD is constitued of two domains S1 (localised juste upstream M1 transmembranaire domain) and S2 and has affinity for L-glutamate [wiki https://fr.wikipedia.org/wiki/Acide_glutamique L-glutamate] or sometime glycine. Positive charge of Amino-group of the agonist bind to negativ charges residue of the pocket D731. In GlurR, negative charge amino acid is a E731 and is able to form salt bridge with agonist. In NR2A D731 is not able to do salt bridge with amino group because aspartate is one methylene lacking to do it. Amino group of agonist is stabilised by water mediated hydrogen bonds to amino acid E413 and Y761. The high affinity for glutamate agonist may be because of van der Walls contact between γ-carboxylate group of glutamate and Y730 of S2 domain which is conserved in NR2 protein. [6]
M2 loop is a channel-lining loop and located in transmembranaire domain. Two asparagines are located on N site of the domain and block Mg2+ and are permeable of Ca2+ [7]
CTD domain is the less conserved of NR2 domain and like ATD allows différents localisation of NMDAr thanks to reticulum endoplasmic trafficking. It is indispensable to receptor surface dynamic and activation of specific signaling. CTD phosphorylation can modulate NMDAr, for instance it is useful for endocytosis during glutamate binding on LBD.[7]
Regulation
NR1/NR2A complex
Mutations
The C-terminal truncation of NR2 subunits in NMDA receptors is an interesting mutation. Indeed, the C-terminal truncation of NR2A influences the functioning of the NMDA receptor. Several experiments were carried out and made it possible to conclude on this influence.
First, mice with truncated NR2A subunits have been shown to still have a functional receptor channel, but NR2A ΔC / ΔC mice appear to be altered in the cellular signal transduction events involved in the induction of LTP (potentiation long-term). Despite the presence of the full-length NR2B subunit, the C-terminal truncation of the NR2A subunit altered the signal transduction mediated by NMDAR.
Second, the C-terminal truncation of NR2A has been shown to alter fear in a particular setting. Indeed, the experiment was to put mutant and wild mice through stepwise avoidance training and put them in water. Thus, the researchers concluded that mutant mice have a significantly reduced latency time to leave the safe platform compared to wild mice and that mutant mice exhibit water balance deficits.
The C-terminal truncation of NR2A therefore has consequences on synaptic plasticity and synaptic reorganization during the recording of hippocampal LTP, on the conditioning of fear and also on motor coordination. [8]
Disease
Anti-nuclear antibodies produced in systemic or systemic lupus erythematosus (SLE, SLE) interact with the NR2A subunit of the NMDA receptor. This is because the Asp / Glu-Trp-Asp / Glu-Tyr-Ser / Gly pentapeptide is a molecular mimic of double-stranded DNA, so antibodies produced in a patient with SLE recognize this pentapeptide. The latter is also present in the structure of NR2A. Thus, these antibodies cross-interact with NR2A and therefore the NMDA receptor. This interaction can signal neuronal death by an excitotoxic mechanism.
More generally, NMDA receptor dysfunction is implicated in multiple brain disorders, such as stroke, chronic pain, and schizophrenia. [9]
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.