Introduction
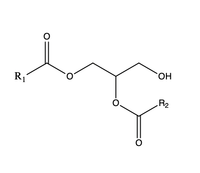
There are two families of DGAT proteins each with their own distinct cellular functions via synthesis of triacylglycerides from oleoyl-CoA. Diacylglycerol acyltransferase 1 (DGAT1) catalyzes the final and only committed step of triacylclygerol synthesis. [1] It does this by using diacylglycerol (DAG) and oleoyl CoA as substrates. DGAT1 is located in the membrane of the endoplasmic reticulum and is important for metabolism through its uptake of diacylglycerides and synthesis of triacylglicerides. [2] This metabolism is involved in intestinal fat absorption, lipoprotein assembly, lactation, and adipose tissue formation [3].
Structural highlights
Conformation
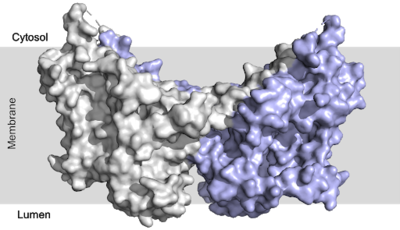
There are three domains within the DGAT enzyme: cytosolic, transmembrane, and luminal. Most of the enzyme exists within the membrane with small portions peeking out into the cytosol of the cell or lumen of the endoplasmic reticulum. DGAT exists as a homodimer of two identical chains, A and B. The homodimer interface is stabilized in two different ways. First, the transmembrane region is stabilized through large between the TM1 helices on each of the chains. The second is through extensive between the two chains in the cytosolic domain. [2] [4]
Active Site
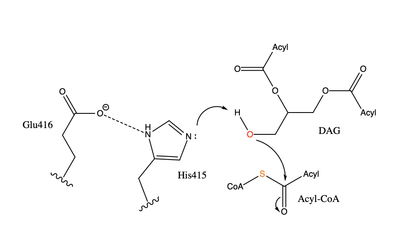
The active site of DGAT is in the transmembrane region of the enzyme. When it is in its state and no oleoyl-CoA is in its tunnel, Met434 hydrogen bonds to the catalytic Histidine, His415, which stabilizes the conformation. There are no major conformation changes that take place upon oleoyl-CoA binding into the cytosolic tunnel, however, several key residues change conformation to allow for the entrance of the ligand. In its conformation, His415 hydrogen bonds to Gln465 which stabilizes the Histidine and allows it to be positioned near the thioester bond of the oleoyl-CoA.[2] The His415 interacts with the DAG that enters in a tunnel perpendicular to the oleoyl-CoA. has been hypothesized to be important in holding the DAG in a proper orientation to be able to interact with the oleoyl-CoA and become a triglyceride. [2]
Tunnel System
There are two main tunnels that allow the enzymatic activity of DGAT to occur. The first is a where the hydrophilic region of the oleyol-CoA binds to the cytosolic face that forms between helices TM6 and TM7. The ]
https://en.wikipedia.org/wiki/Coenzyme_A CoA] region sits at the cytosolic face with the fatty acid chain extending the rest of the way through the enzyme tunnel.
[4] Researchers are currently unsure of specifically what residues are involved in the binding of the oleoyl-coA to the reaction chamber, but they believe that the hydrogen bonding of the coenzyme A motif that sits at the cytosolic face of the tunnel. When hydrophobic residues were substituted with the standard residues in the cytosolic tunnel, DGAT was completely inactivated.
[4] [2]
The second is a that is orthogonal to the cytosolic tunnel. This tunnel is in the transmembrane region of the enzyme which allows lipids in the membrane to easily access the location.
[2] Researchers have hypothesized that this tunnel is able to differentiate between DAG, its intended substrate, from other groups that would typically interact with an acyl-CoA, like cholesterol, due to its bent architecture. The bent nature of the tunnel would inhibit more stiff and planar molecules from entering the tunnel and interfering with the activity of the enzyme.
[2]
Mechanism
When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, the transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride. The deprotonates the hydroxyl group on the C3 of the glycerol backbone. The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and back down to the carbonyl carbon. The sulfur of the Acyl-CoA takes the added electron density and the bond between the sulfur and carbonyl carbon is broken. Glu416 likely provides transition state stabilization of the His415
[2] despite being outside of the 3 angstrom hydrogen bonding distance. This is likely due to the fact that the DGAT 1 enzyme was unable to be visualized with the diacylglycerol in the active site. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 support the hypothesis that it is essential for stabilization in the active site since the enzyme function was completely eliminated when the mutation was made.
While there is not universal agreement on whether provide transition state stabilization for the acyl transfer activity of the enzyme, it is believed that Glu416 is more likely to provide stabilization due to positive findings upon mutations of that residue. Point mutations were made to various residues in the active site including Glu416. Mutations to the entrance of the binding site have a smaller impact on the functionality of the enzyme than mutation to the rest of the active site, while mutations to His415 and Glu416 abolish enzymatic activity completely. This suggests that His415 and Glu416 are essential to the reaction whereas Gln465 can be changed without significantly impacting the functionality of the enzyme.