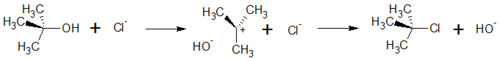
In general, substitutions exchange substituents in an organic molecule. One example of an SN1 reactions are the exchange of the Hydroxide in tert-Butanol by a Chloride Ion or
In general, SN1 substitution can take place when a stable carbocation can be formed. If not, the reaction follows the SN2 mechanism.
The SN1 with the removement of a hydroxide-ion out of the molecule, in this case tert-Butanol. By this, a positively charged carbocation with a planar geometry is formed. This step is also the rate-determing step because it is the slowest step in this reaction.
In the , the haloanion bound to the carbocation, and a neutral haloalkane is formed. With this step, the hydroxy-substituent is replaced by a halogen-substituent.
See also
SN2 reaction: Substitution of chloride and methanol
References
This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn1_substutition/sn1_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project