This is a default text for your page George G. Papadeas/Sandbox VKOR. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
History of VKOR
Function and Biological Role
Author's Notes
Structural Highlights
Active Site
Cap Domain
Closed conformation
Anchor
Function: Method of Coagulation
Brief Overview
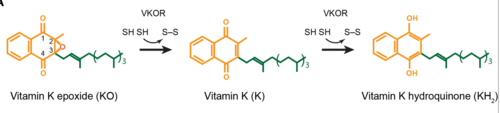
Catalytic Mechanism
The catalytic mechanism of VKOR is a critical part of its overall function in the body. Highly regulated enzymatic activity through the reactivity of catalytic cysteines allows VKOR to properly activate Vitamin K for its use in the body. The enzyme begins in STAGE 1, where it's in the open conformation with the cap domain open to allow in a substrate to bind to the active site. Once a substrate binds, the cap domain is initiated into the closed conformation. VKOR is now in STAGE 2. To further stabilize the closed conformation with the substrate bound, the cap domain helps initiate a catalytic reaction of cysteines to break the disulfide bridge that was stabilizing stage 1. Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in STAGE 3, where the catalytic free cysteines react to form a new disulfide bridge, releasing the activated substrate into the blood stream to promote anticoagulation. With two stable disulfide bridges and VKOR unbound, the enzyme is now in its final, unreactive STAGE 4. VKOR must undergo conformational changes to return to Stage 1 and restart the catalytic process to activate Vitamin K again.
Disease and Treatment
Afflictions
Inhibition
Mutations
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.