Sandbox Reserved 1701
From Proteopedia
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
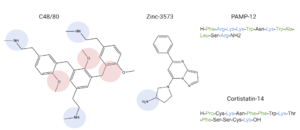
BackgroundLigandsMRGPRX2 binds a wide range of small molecule and peptide ligands. These ligands have positively charged regions which can interact with the negative binding pocket, sub-pocket 1. Some of these larger ligands also have a hydrophobic region which can interact with sub-pocket 2. MRGPRX2 has been known to interact with PAMP-12, Cortistatin-14, C48/80, and Zinc-3573[1],[2].
Ligands' charge or hydrophilicity are shown to demonstrate where they may interact with sub-pockets 1 and 2. , specifically, interacts with sub-pocket 1 through LYS-3 and sub-pocket 2 through LYS-8. These ligands also share similar structural features with targeted drugs for clinical relevance. MRGPRX2 has been identified as a potential target for drugs such as Atracurium, Rocuronium, Ciprofloxacin, and Levofloxacin[3]. These drugs have similar structural regions which may interact with sub-pockets 1 and/or 2. SignificanceStructureHeterotrimeric G-Protein Structure
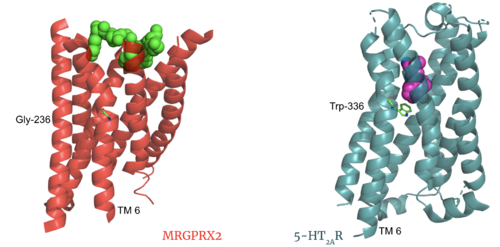
Novel CharacteristicsMRGPRX2 demonstrates novel characteristics compared to other class A GPCRs. These structural motif differences contribute to a surface ligand binding rather than a ligand binding deep within the helices. To demonstrate this difference in depth binding, MRGPRX2 is compared to 5-HT2AR, another class A GPCR with more conserved structural motifs.
Toggle SwitchPIF/LLF MotifDRY/ ERC MotifMRGPRX2 has an rather than the typically conserved E/DRY Motif. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart.
Sodium SiteThe MRGPRX2 consists of ASP-75 and GLY-116 compared to the previously conserved residues in this binding pocket. Other class A GPCRs demonstrate a larger binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this pocket lacks the same amount of with the shift to a glycine residue rather than typical negative residues. The helices for the MRGPRX2 in the binding pocket are also more collapsed making this pocket less accessible for sodium ions. Disulfide BondsThe MRGPRX2 disulfide bond is between CYS-168 and CYS-180 on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands.
Further InformationThe MRGPRX2 GPCR also contains semi-conserved or fully-conserved motifs that are seen in other class A GPCRs. NPxxY MotifThe residues in the are pivotal for receptor activation in all Class A GPCRs. This motif is conserved in the MRGPRX2 receptor with residues VAL-231, ASP-75, ASN-275, and TYR-279. CWxP MotifThe is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved. CYS6.47 may play a fundamental role in GPCR activity which would be supported by its conservation in MRGPRX2[4]. FunctionGPCRs undergo a transmembrane protein conformational change upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses. Before ActivationThe culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding.The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding. This contrast is represented in the photo below to show the unbound and bound transmembrane proteins of MRGPRX2 and 5-HT2A. After ActivationAfter ligand binding and transmembrane protein activation, this signal is sent to the alpha subunit of the G-protein which undergoes its own chemical and conformational changes. The alpha subunit is initially bound with GDP which then is physically replaced by GTP leading to conformational changes. These changes can be seen in this video of a G-protein alpha subunit activation derived from common rats. Clinical Relevance3D StructuresReferences
| ||||||||||||