Function
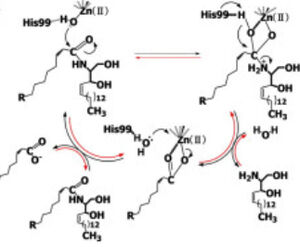
Figure 1 Proposed mechanisms of the zinc-dependent hydrolysis of C2-ceramide (Black arrows) and the zinc-dependent synthesis of C2-ceramide from palmitate and sphingosine (Red arrows) by CerN . Figure adapted from Inoe et al.(2009)
[1] CerN is an enzyme that catalyzes the cleavage of the Sphingolipid at the , producing .[2] [1] As a neutral ceramidase, optimal catalytic activity of CerN occurs between pH 6.5-8.5.[2] CerN cleaves the N-acyl linkage within ceramides via zinc-dependent hydrolysis and the enzyme is also capable of synthesizing ceramide from sphingosine and palmitic acid by the reverse mechanism.[1][3] The zinc ion within the is coordinated by His97, His204, Glu411, Tyr448, and a water molecule. His97 and Tyr448 are required for zinc binding within the active site. Ligand binding within the active site is recognized by Gly25, His99, Arg160, and Tyr460.[1] Ser27 and Gly25 stabilize ceramide within the active site by forming a water-mediated hydrogen bond with the central OH of ceramide, and the carbonyl oxygen is stabilized by the Tyr448 and Tyr460.
Upon ligand binding, CerN enters the conformation. [1] His99 and Arg160 function in the catalysis of ceramide hydrolysis, as they deprotonate their coordinated water molecule to produce a hydroxide ion.[1] The carbonyl carbon of ceramide undergoes a nucleophilic attack by the hydroxide ion (Figure 1).[1] The carbonyl oxygen stabilized by Tyr448 and Tyr460 is then passed to the zinc ion, allowing for the breakage of the N-acyl linkage.[1] Sphingosine is then released from the active site while the fatty acid remains bound to the zinc ion until it is replaced by a new water molecule, shifting CerN into the conformation.[1] The synthesis of ceramide from palmitate and sphingosine occurs via the same mechanism but in reverse (Figure 1). [1]
Refs [2], [1] , [3]
Structural highlights
CerN consists of two domains: a .[1] Three β-sheets, each formed from four β-strands, compose a β-prism fold at the center of the N-terminal domain.[1] Surrounding the β-prism fold are 11 α-helices, forming an .[1] The immunoglobulin-like C-terminal domain is composed of two β-sheets, containing four β-strands each, forming a .[1] Between the N- and C-terminal domains is a magnesium/calcium ion binding site that links together the two domains.[1] His37, Asp579, Asp581, and Thr854 interact with divalent cations within the .[1] A is located within the N-terminal domain active site, where it is coordinated by His97, His204, Glu411, and a water molecule.[1]
Full crystallographic information for 2zxc (Closed) is available from OCA.Full crystallographic information for 2zws (Open) is available from OCA. For a guided tour on the structure components use FirstGlance.
|
| Ligands: | , , , , |
| Activity: | Ceramidase, with EC number 3.5.1.23 |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT |
Role of Ceramide and CerN in Bacterial Infections
Sphingolipids play key role in eukaryotic cell membrane structure and function.[4][5] Additionally, sphingolipids act as signaling molecules for eukaryotic processes such as proliferation, apoptosis, inflammation, cell migration, and pathogen defense.[5] Ceramide is considered as the central molecule in sphingolipid metabolism, as it can be converted to more complex sphingolipids or be broken down for the production of sphingosine and sphingosine-1-phosphate.[6] [7] [8] Eukaryotes utilize ceramide in the formation of lipid rafts, lipid-protein platforms that alter the biophysical properties of cell membranes as well as localize receptors for signal amplification.[5] [7] Sphingosine and sphingosine-1-phosphate, the products of ceramide catabolism, are important for pathogen defense by functioning as a potent anti-microbial and activating signal for host-immune responses, respectively.[6]
Due to its abundance in eukaryotic cell membranes and role in pathogen defense, pathogens have evolved mechanisms to use host ceramides for their own pathogenesis.[7] Ceramide-rich membrane platforms on human-epithelial cells serve as sites for the attachment and invasion of bacterial pathogens such as Neisseria gonorrhoeae.[7] Pseudomonas aeruginosa is capable of detecting host-derived sphingosine, resulting in activation of P. aeruginosa sphingosine-responsive genes.[9] The products of P. aeruginosa sphingosine-responsive genes are used for the detoxification of sphingosine, as well as its production from other host-derived sphingolipids, making P. aeruginosa sphingosine tolerant.[9] Loss of P. aeruginosa sphingosine-responsive genes results in the inability of the bacteria to survive in the presence of sphingosine in vitro or in the murine lung.[9] CerN is one of the proteins encoded by P. aeruginosa sphingosine-responsive genes used for the production of sphingosine from ceramide, with the added ability of functioning as a ceramide synthase.[2][3][9] It has been hypothesized that the hydrolysis of host-membrane ceramide via CerN facilitates P. aeruginosa intracellular invasion, similar to other bacterial pathogens.[2] CerN is also involved in P. aeruginosa biofilm production, a major virulence trait of the pathogen.[10] Sphingosine production via CerN-mediated hydrolysis of host ceramide induces a biofilm formation at sites of infection, biofilm accumulation yields more ceramide hydrolysis, creating a positive-feedback loop for P. aeruginosa virulence.[10]
Relevance
p

