Sandbox reserved 1753
From Proteopedia
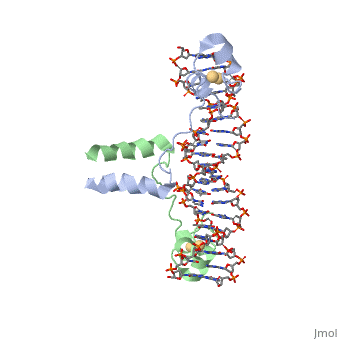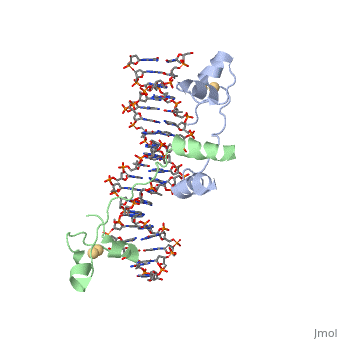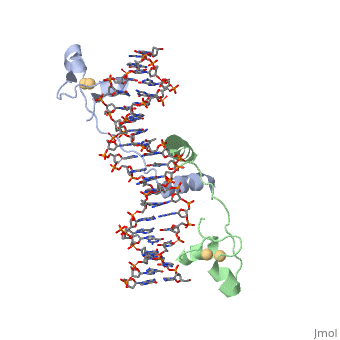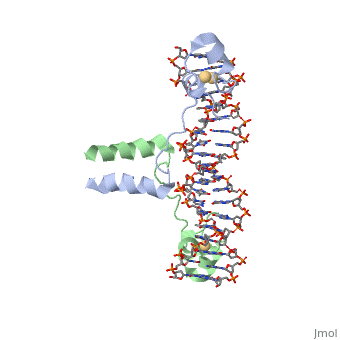
==DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX==
Structural highlights
Function[GAL4_YEAST] This protein is a positive regulator for the gene expression of the galactose-induced genes such as GAL1, GAL2, GAL7, GAL10, and MEL1 which code for the enzymes used to convert galactose to glucose. It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedA specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a to a symmetrical 17-base-pair sequence. Each subunit folds into three distinct modules: a compact, (residues 8-40), an (41-49), and an (50-64). A small, -containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4.
DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||
A NUCLEOTIDE-FLIPPING MECHANISM FROM THE STRUCTURE OF HUMAN URACIL-DNA GLYCOSYLASE BOUND TO DNA
Structural highlights
Disease[UNG_HUMAN] Defects in UNG are a cause of immunodeficiency with hyper-IgM type 5 (HIGM5) [MIM:608106]. A rare immunodeficiency syndrome characterized by normal or elevated serum IgM levels with absence of IgG, IgA, and IgE. It results in a profound susceptibility to bacterial infections.[1] [2] Function[UNG_HUMAN] Excises uracil residues from the DNA which can arise as a result of misincorporation of dUMP residues by DNA polymerase or due to deamination of cytosine. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedAny bases in DNA, a result of either misincorporation or deamination of cytosine, are removed by uracil-DNA glycosylase (UDG), one of the most efficient and specific of the base-excision DNA-repair enzymes. Crystal structures of human and viral UDGs complexed with free uracil have indicated that the enzyme binds an extrahelical uracil. Such binding of undamaged extrahelical bases has been seen in the structures of two bacterial methyltransferases and bacteriophage T4 endonuclease V. Here we characterize the DNA binding and kinetics of several engineered human UDG mutants and present the crystal structure of one of these, which to our knowledge represents the first structure of any eukaryotic DNA repair enzyme in complex with its damaged, target DNA. Electrostatic orientation along the UDG active site, insertion of an amino acid (residue 272) into the DNA through the minor groove, and compression of the DNA backbone flanking the uracil all result in the flipping-out of the damaged base from the DNA major groove, allowing specific recognition of its phosphate, deoxyribose and uracil moieties. Our structure thus provides a view of a productive complex specific for cleavage of uracil from DNA and also reveals the basis for the enzyme-assisted nucleotide flipping by this critical DNA-repair enzyme. IntroductionGlycosylase is an enzyme. Its main function is in Base Excision Repair(BER) it removes and repairs damaged bases usually these are single stranded DNA breaks. BER corrects DNA damage that resulted from small leisures that do not disrupt the double helix. The way Glycosylase does this is by first cleaving the glycosidic bond of the damaged nucleotide leaving the Deoxyribose nucleotide with no base. The deoxyribose is then cleaved as well by AP endonuclease. The gap that is left is filled in through DNA Polymerase and DNA ligase. The structure of Glycosylase has a couple different forms in terms of its general structure. It is composed of a 10bp DNA that contains U G base pair mismatch. This is what allows it to bind the DNA flipping them out of the double helix. When the uracil mismatch is flipped out of the helix an takes its place. The actual structure is composed of a protein section bound to a DNA section. This is often represented by showing the DNA section in stick form and coloring it based on the different atoms bound. The protein section is characterized using a ribbon diagram. In our biochemistry book page 897 there is a representation of this as well.
A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA.,Slupphaug G, Mol CD, Kavli B, Arvai AS, Krokan HE, Tainer JA Nature. 1996 Nov 7;384(6604):87-92. PMID:8900285[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||||
Categories: Atcc 18824 | Large Structures | Carey, M | Harrison, S C | Marmorstein, R | Ptashne, M | Double helix | Protein-dna complex | Transcription-dna complex | Human | Uridine nucleosidase | Arvai, A S | Kavli, B | Krokan, H E | Mol, C D | Slupphaug, G | Tainer, J A | Dna | Dna base excision repair | Dna glycosylase | Hydrolase-dna complex | Uracil