Sandbox Reserved 1771
From Proteopedia
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
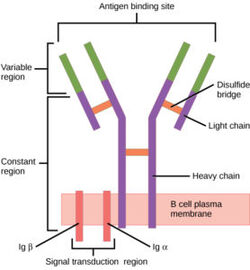
IgM B-cell Receptor
Introduction
The human B-cell receptor(BCR) is a complex protein made up of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein. BCRs are found on the surface of B-cells as membrane bound proteins (ref). In general the role of BCRs is to bind to foreign antigens and initiate the appropriate immune response.
Structure
| |||||||||||
Function
Once bound to an antigen, BCRs undergo a conformational change in the extracellular region. While the exact conformational change is still not known, it initiates several signal transduction pathways. These pathways are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an immunoreceptor tyrosine-based activation motif(ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response. BCRs can be oligomeric prior to antigen binding, but once bound become an active monomer.
Medical Relevancy
B-cell Formation
The formation of B-cells occurs in the bone marrow. BCRs are attached to B-cells through the aid of membrane-bound proteins in bone marrow cells. During this process, gene recombination occurs, which allows unique BCRs to become highly specific to different antigens.