Active and Inactive Form
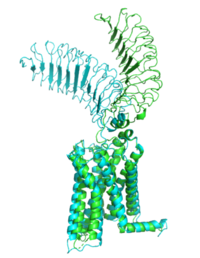
Figure 1: Inactive form of the thyrotropin receptor shown in blue. The active form of the thyrotropin receptor is shown in green.
The TSHR protein exists in two states, active and inactive (Figure 1). The extracellular domain sticks out from the cell membrane into the space outside the cell. The contains 7 alpha helices that reside within the cell membrane. The exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the upstate, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of a clash with the cell membrane.[1] Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. [1]
TSHR Agonists and Inverse Agonists
Chemical agonists are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical inverse agonists bind to the same receptor as the agonist but promotes a biological response opposite that of the agonist. Different types of agonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead researchers to uncover the receptor's binding mechanism.
M22 Agonist
is a
monoclonal antibody that was isolated from a patient with
Graves' Disease. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity.
[2]. The M22
autoantibody activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR.
[1] Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.
[1] This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation.
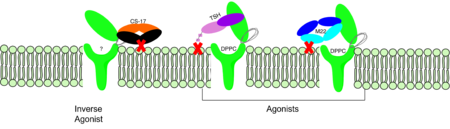
Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.
CS-17 Inverse Agonist
CS-17 (GREEN LINK) is a monoclonal antibody that acts as an inverse agonist for TSHR constitutive activity. [3]. CS-17 interacts with the extracellular domain of the TSHR protein on the convex side of the LRRD. When bound to TSHR, CS-17 suppresses TSHR function by keeping the receptor in the inactive state (Figure 2). Clash with the cell membrane does not allow the inactive form of TSHR to flip to the active conformation. CS-17 plays a unique role with GPCRs. This type of inhibition is not commonly seen in many biological systems and therefore leads to this method of inhibition being a great target for drug design and future research.[3]. Due to its unique inhibition, CS-17 can be a popular therapy for many thyroid diseases where the thyroid is overactive.
TSH Agonist
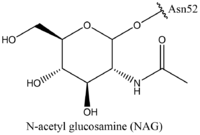
Thyroid-stimulating hormone (TSH) (GREEN LINK) is a hormone that stimulates the thyroid gland to produce proteins that are vital for many metabolic pathways in the body's tissue. TSH activates the TSHR protein by binding to the concave surface of the LRRD and hinge region to keep TSHR in its active state by clashing with the membrane [4]. (Figure 2). This clash is caused by glycosylations of an asparagine 52 residue on the alpha subunit of TSH (GREEN LINK). These modifications to the ASN residue are N-acetyl glucosamine modifications (Figure 3). They stick out from the alpha subunit of TSH to clash with the cell membrane and keep TSH in the active state.
ML109