Introduction
SHOC2-PP1C-MRAS is a regulatory protein and enzyme complex that is involved in regulating cell proliferation and division [1]. This complex regulates the vast RAS-MAPK signaling pathway through control of the initial RAF MAPKKK. This pathway is initially activated by the binding of a growth factor to a GTPase which initiates intracellular RAS activation, such as HRAS, NRAS, or KRAS.
[1]. RAS-GTPases are a family of monomeric G-proteins that function as molecular switches and are key in regulatory parts in signaling cascades. The RAS molecular switch alterantes between binding GTP for the active state and GDP to become inactive [1].

Figure 1. Schematic representation of RAS/RAF/MEK/ERK pathway after assembly of SHOC2-PP1C-MRAS complex.
After activation via an extracellular growth factor, the RAS-GTPase enzyme binds GTP, which recruits RAF to the cell membrane (more in depth here?).RAF[2] is a kinase that stimulates a signaling cascade by phosphorylation of MAPK (also known as MEK in mammals), which activates downstream proteins such as ERK 1 and ERK2, which subsequently activate nuclear transcription factors and kinases (give examples?). The RAS/RAF/MEK/ERK pathway is a critical signaling cascade for activating transcription factors and regulating gene expression[3].
Structure
Overview
The protein combines three separate (see notes for more). SHOC2, PP1C, and MRAS, to form the active protein (SMP complex), as seen in Figure 2[4]. The SMP complex was determined via cryo-electron microscopy as well as x-ray diffraction. The overall architecture has PP1C and MRAS bound within the concave surface of SHOC2, leaving the catalytic site of PP1C and the GTP binding cleft in MRAS exposed.
SHOC2
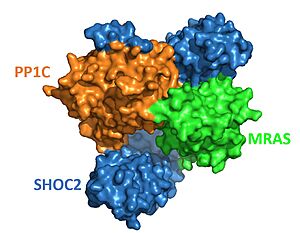
Figure 2. Surface representation of SHOC2-PP1C-MRAS (
PDB 7upi). SHOC2 (blue), PP1C (orange) and MRAS (green).
SHOC2 is a scaffolding protein which acts as a cradle to bind PP1C and MRAS [4], serving as an aggregation point to enable reciprocal interactions between these 3 signaling proteins. is a leucine rich repeat (LRR) protein consisting of 20 consecutive . LRR motifs form an an extended β-sheet on the inner concave surface of SHOC2 with α-helices facing outward to facilitate binding of the protein complex. These LRR motifs result in a largely hydrophobic core within the concaved region.[5].
PP1C
is the catalytic domain of the phosphatase enzyme PP1, which removes reversible phosphorylations from signaling proteins. PP1C is a serine/threonine phosphatase involved in signaling pathways that control cell growth, division, and metabolism (REF) and explain more, see notes.
MRAS
is a monomeric GTPase and is anchored in the cell membrane. When MRAS binds GTP, it becomes active, and can only bind the complex once activated [4]. MRAS is a subvariant of the RAS protein and therefore shares most of it's regulatory and effector interactions[6]. While other RAS variants bind in complex with SHOC2 and PP1 to allow it to have phosphatase activity, MRAS binds the tightest.
Switch I and II
A key feature in allowing MRAS to be in its active vs inactive state is MRAS' switches. When MRAS is active GTP is bound and the switches allow MRAS to be bound with PP1C. When MRAS is inactive GDP is bound and the switches do not allow MRAS to bind to PP1C. The switches determine whether MRAS can bind to SHOC2-PP1C. The switches have to go through a conformational change to allow binding of SHOC2-PP1C to MRAS. The conformational change is needed because without it SHOC2-PP1C could bind to MRAS when MRAS is still inactive. This process would cause the SHOC2-PP1C-MRAS pathway to constantly be running. The switches and GDP/GTP help regulate this process.
This conformational change is caused by GTP replacing GDP. When GTP is bound, MRAS shifts and binds with the previously associated SHOC2-PP1C complex. When GDP is bound, switch II in MRAS is moved outward, which causes a steric clash with SHOC2 . When GTP is bound, switch II in MRAS can form various hydrogen bonding, pi stacking and hydrophobic interactions with SHOC2[7] . When MRAS is bound to SHOC2-PP1C, switch I has an important role in making interactions with PP1C.
Stabilizing Interactions in Ternary Complex
exclusively through its concave LRRs[8], primarily by the descending loop and strands of LRR domains 2-10. Add his sentence thing with the ascending loops of the SHOC2 LRR regions, and is further engaged through the N-terminal region of SHOC2 containing the [9]. The initial forming of the complex begins with SHOC2-PP1C engagement, then is completed and stabilized by the GTP-loaded MRAS binding[9]. Once associated with SHOC2, . Binding to MRAS localizes the other two proteins to the RAS signaling regions of the membrane to begin cellular signaling[8].
Active Site
Once all the domains are bound NTpS binds to PP1C in the active site. Once NTpS is bound it becomes dephosphorylated and the complex falls apart. NTpS is dephosphorylated to prevent the active dimeric RAF from inactivating and changing into its monomeric structure. The is surrounded by hydrophobic and acidic regions along with the C-terminus. These regions are located on the surface of PP1C whereas the active site is placed further into the structure. It is thought that these regions help the ligand bind to the active site by making interactions that will lead NTpS into the protein. There is still some uncertainty as to how the substrate selectivity works but these regions could play an essential role in it. Specifically, the pS from NTpS would bind to the hydrophobic region on PP1C[10].
NTpS can bind to the PP1C active site without PP1C also being bound to SHOC2 and MRAS, however the catalytic activity is much slower and the reaction is less efficient. Also for this event to occur NTpS would need to be exposed from its binding site in the inactive RAF complex. A RAS has to bind to RAF to expose the NTpS allowing PP1C and NTpS to bind.
let me know thought on the style of image
Activation of RAF
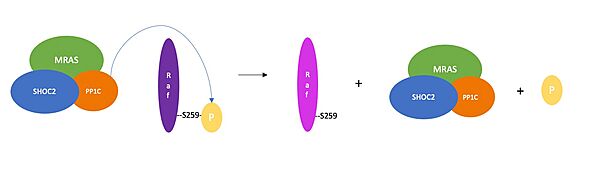
Figure 3. Schematic representation of RAF activation.
Once the SMP complex comes together, it plays a key role in regulating the activation of the RAF and MAPK pathway. To do so, PP1C, enhanced through the interactions with SHOC2 and MRAS, phosphorylates serine 259 on RAF kinases. Doing so regulates cell growth, survival, proliferation, and differentiation [11].
Implications
The SHOC2-MRAS-PP1C complex's key role in the regulation of the MAPK-RAF pathway means that minor changes in its structure or function can have drastic biological consequences. Unregulated activation of the MAPK pathway is the cause of several human cancers due to unchecked cell division and proliferation[8]. Mutations that stabilize the interactions of the SMP complex enhances PP1C phosphatase activity [9], leading to increased RAF signaling and accelerated cell division. Most SHOC2 or PP1C mutations alter residues that are the direct contact points, stabilizing the interaction between the two proteins. Mutations to MRAS result in persistent binding of GTP, leading to consistent activation of the cell signaling pathway[8].
The unregulated MAPK pathway is also responsible for a multitude of developmental disorders commonly known as RASopathies [11]. One such RASopathy caused by the mutation of a RAF kinase is known as Noonan Syndrome (NS), which enhances complex formation by stabilizing the interactions of each member[8]. NS is a genetic disorder that can prevent normal development during the neonatal period, leading to difficulties with feeding and a failure to thrive[12]. The characteristic features of NS become more evident during infancy and childhood. NS patients often have atypical facial appearance, short stature, heart defects, and other physical problems[12].



