User:Francielle Aguiar Gomes/Sandbox 1
From Proteopedia
Photosynthetic LH1-RC Super-complex of Rhodospirillum rubrum
IntroductionRhodospirillum (Rsp.) rubrum is an anoxygenic phototrophic purple bacterium with a long history as a model for the study of bacterial photosynthesis and related metabolic processes. It is unique among purple bacteria by producing both rhodoquinone (RQ) and ubiquinone (UQ)1 as electron carriers and bacteriochlorophyll (BChl) a esterified at the propionic acid side chain by geranylgeraniol (abbreviated as BChlaG) rather than phytol. The light-harvesting complexes (LHC) of photosynthetic purple sulfur and non-sulfur bacteria are responsible for the highly efficient collection and transfer of light energy to the photosynthetic reaction centres. This results in an initial separation of charge in the reaction centre (RC) and ultimately conversion of the light energy into a chemically useful form [1]. Rsp. rubrum has a single pair of αβ-polypeptides in its core light-harvesting (LH1) complex and lacks both the peripheral light-harvesting (LH2) complex and reaction center (RC) cytochrome (Cyt) c subunit present in many purple bacteria; thus, Rsp. rubrum is one of the simplest phototrophic bacteria known, in terms of its photosynthetic light reactions. Because the entire Rsp. rubrum LH1 complex and a stable B820 LH1-subunit can be reconstituted using the αβ-polypeptides and pigment molecules,3−5 both complexes have been intensively studied as models of the bacterial antenna apparatus6 and as such have provided a wealth of information on mechanisms of light energy acquisition, pigment−protein interactions, and assembly of multicomponent complexes. [2] Inicial StructuresStructures of both purified LH1 and the RC-associated core complex (LH1-RC) of Rsp. rubrum have not been obtained at high resolution, and no RC atomic structure was known. The 8.5 Å resolution projection of R. rubrum LHCl represents the first glimpse of the structural architecture of the fundamental building block of the photosynthetic membrane in purple BChla-containing bacteria. The crystals diffract beyond 8 Å and the projection map was calculated to 8.5 A. The projection map shows 16 subunits in a 116 Å diameter ring with a 68 Å hole in the center. 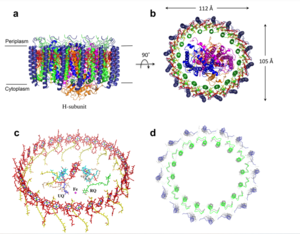 Fig. 1. Structure overview of the Rsp. rubrum LH1-RC complex. (a) Side view of the LH1-RC parallel to the membrane plane. (b) Top view of the LH1-RC from the periplasmic side of the membrane. (c) Tilted view of the cofactor arrangement. (d) Superposition of Cα carbons of the LH1 αβpolypeptides between Rsp. rubrum and Tch. tepidum (gray, PDB: 5Y5S). Color scheme: LH1-α, green; LH1-β, slate-blue; L-subunit, magenta; Msubunit, blue; BChl aG in LH1 and special pair, red sticks; Accessory BChl aG, cyan sticks; BPhe aG, light-pink sticks; Spirilloxanthin, yellow sticks; UQ10, blue sticks; RQ-10, green sticks; Fe, magenta ball. Phospholipids and detergents are omitted for clarity Cryo-EMCryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied to samples cooled to cryogenic temperatures. For biological samples, structure is preserved by embedding in a glassy ice environment. An aqueous sample is applied to a mesh grid and frozen by immersion in liquid ethane or a mixture of liquid ethane and propane [3]. This technique has advanced dramatically to become a viable tool for high-resolution structural biology research. The ultimate outcome of a cryoEM study is an atomic model of a macromolecule or its complex with interacting partners. Recent advances in direct electron detectors as well as reconstruction single particle algorithms have led to the determination of the structure of macromolecular complexes ranging from 2 to 5 Å resolution. At these resolutions, also known as “near atomic” resolution, it is possible to infer all-atom structures de novo.
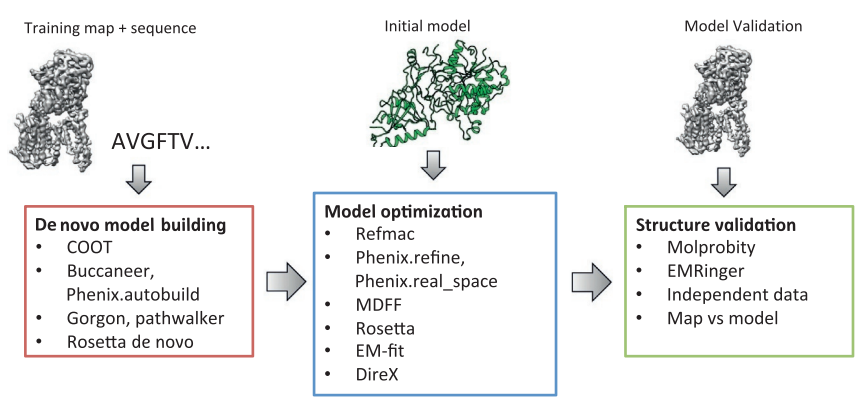
The first step in cryoEM structure determination is de novo structure determination, where an initial model can be built, given only one sequence and a reconstruction, when no other limited structural information is known. In the second stage, the model is optimized, where a wide range of class of methods for improving the fit of a model to the data and improving the geometry of a model. Finally, tools for model validation are described, in attempt to quantify the overall accuracy of a model given a reconstruction.
Structure of Photosynthetic LH1-RC Super-complex of Rhodospirillum rubrumThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. References | ||||||||||||