Structural highlights
Publication Abstract from PubMed
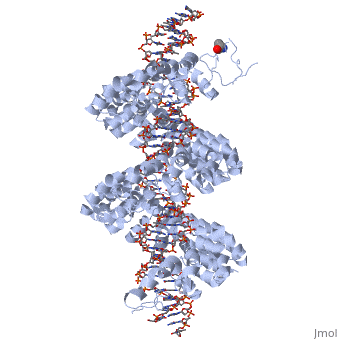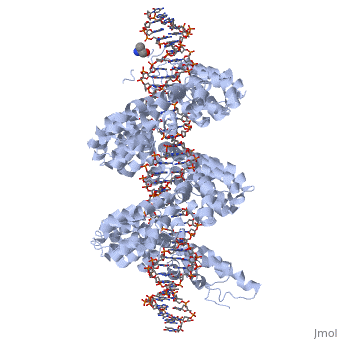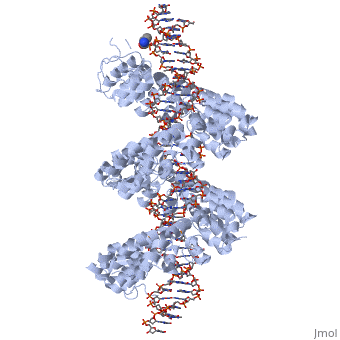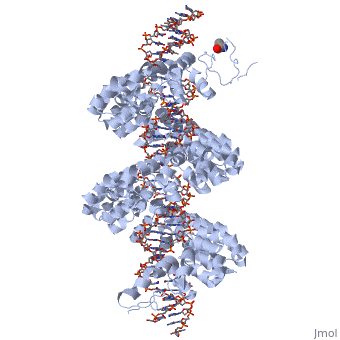
DNA recognition by TAL effectors is mediated by tandem repeats, each 33 to 35 residues in length, that specify nucleotides via unique repeat variable diresidues (RVDs). The crystal structure of PthXo1 bound to its DNA target was determined using high-throughput computational structure prediction and validated by heavy-atom derivatization. Each repeat forms a left-handed, two-helix bundle that presents an RVD-containing loop to the DNA. The repeats self-associate to form a right-handed superhelix wrapped around the DNA major groove. The first RVD residue forms a stabilizing contact with the protein backbone, while the second makes a base-specific contact to the DNA sense strand. Two degenerate N-terminal repeats also interact with the DNA. Containing several RVDs and noncanonical associations, the structure illustrates the basis of TAL effector-DNA recognition.
The Crystal Structure of TAL Effector PthXo1 Bound to Its DNA Target.,Mak AN, Bradley P, Cernadas RA, Bogdanove AJ, Stoddard BL Science. 2012 Jan 5. PMID:22223736[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Mak AN, Bradley P, Cernadas RA, Bogdanove AJ, Stoddard BL. The Crystal Structure of TAL Effector PthXo1 Bound to Its DNA Target. Science. 2012 Jan 5. PMID:22223736 doi:10.1126/science.1216211