This is a default text for your page Ben Whiteside. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
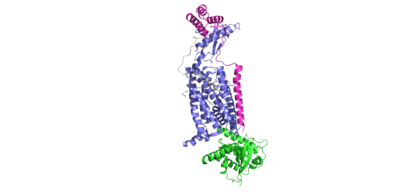
When consuming food, the human body is tasked with secreting hormones and chemical messengers that will help regulate homeostasis. After a meal, our body has to maintain homeostasis by reducing our blood glucose and signaling that we have consumed enough nutrients. During feeding, cells in the body will secrete the ligand, amylin. Amylin is a 37 amino acid glucoregulatory hormone that is produced within beta cells of the pancreas. When there is an influx of nutrients in the gastrointestinal tract, the ligand will bind to the heterodimeric receptor, activating the receptor and triggering the corresponding signaling cascade. The overall effect of this cascade is increased satiety, delayed gastric emptying, and inhibition of glucagon secretion. The amylin receptors are widely distributed throughout the central nervous system. The is a heterodimeric protein containing a calcitonin receptor domain, as well as one of three receptor activity modifying proteins(RAMP 1,2, or 3).
[3]
[4]
Amylin Peptide
Transmembrane Domain
Within the transmembrane domain of the CTR, hydrophobic R groups span the phospholipid bilayer, anchoring the protein into the cell membrane upon amylin binding to the receptor.
Chemical Modifications to Amylin
N-Terminus Disulfide
The amylin peptide contains a between residues C2 and C7. This disulfide provides stability and rigidity to the helical structure of the peptide, allowing for favorable binding to the extracellular domain (ECD).
Amidated C-Terminus
The C-terminus of amylin contains an amide group, rather than a carboxylic acid group. This chemical modification allows for more extensive hydrogen bonding to nearby residues, due to the added hydrogen bond donor on the NH2 group. In turn, this allows for favorable hydrogen bonds between S129 of the transmembrane domain and the main chain of Y37 on amylin. This interaction causes a "kink" in the random coil of amylin, displacing Y37 into a hydrophobic pocket, allowing for favorable hydrophobic interactions with W79 of the transmembrane domain.
Receptor Activity Modifying Proteins
is a key interaction that stabilizes the protein complex and positions the receptor to favorably bind to amylin. The RAMP-CTR interface extends into the plasma membrane, providing additional non-covalent bonding between the protein complex and the cell membrane.
Extracellular Domain - RAMP interactions
The extracellular domain of the CTR primarily contains polar residues in the extracellular space. In order to orient these residues in such a way to facilitate amylin binding, RAMP makes hydrogen bonds with the CTR to increase the rigidity of the receptor binding site.
Bypass Motif
G-alpha Interactions with CTR TMD
To transduce the signal across the cell membrane, the binding of amylin will induce a conformational change that allows for the CTR to make favorable interactions with the G alpha subunit. Two interactions shown and activate the G-protein and propel downstream signaling.
Function
Relevance