Introduction
History
Interest in pancreatic hormones began at the turn of the 20th century. In 1902, Bayliss and Starling discover a pancreatic secretin involved in regulation of water homeostasis, giving rise to interest in pancreatic hormones. Shortly after, in 1906, Moore et al. hypothesize involvement of gastrointestinal hormone extracts in maintenance of the endocrine pancreas. Jean La Barre purifies glucose-lowering gut extracts in 1929 and characterizes them as incretins, which is short for intestine secretion insulin. Finally, in 1984, gastric inhibitory peptide (GIP) is isolated from porcupine intestine. Although GIP was initially characterized for its gastric inhibitory effects (hence the name), it was also shown that the polypeptide played an integral role in insulin signaling and secretion. Interestingly, it was found that the effect of GIP on insulin levels was still seen in its absence, hinting toward the presence of an additional incretin, which has now been classified as glucagon-like peptide-1 (GLP-1).
General Structure
Subunit Architecture
Glucose-dependent Insulinotropic Polypeptide
Active Site
Associated Diseases
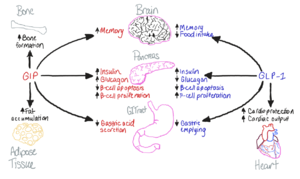
Figure 1. The biological roles of GIP and and GLP-1, incretin hormones.
Medical Relevance
Tirzepatide

Figure 2. Sequence alignment of GLP-1, GIP, and Tirzepatide. Residues shown in black are found in all three amino acid sequences. Pink or red coloration denotes residues that are unique to GIP or GLP-1, respectively. The sequence of tirzepatide is colored accordingly, and residues differing from both GIP and GLP-1 are highlighted in blue. Residues that differ across all three structures are boxed.


