Background
Plastic pollution is a major problem in our modern world. Many strategies have been employed to recycle plastics instead of creating more and adding further pollution to the environment, as displayed in Figure 1.

Figure 1 - A real world example plastic and the evident problem it possesses
One of the most commonly used plastics is a polymer called Polyethylene Terephthalate, also known as PET. PET is commonly used to make plastic bottles or food containers due to its thermal stability, transparency, and impermeability to liquids and gases[1]. Unfortunately, these properties that make it desirable also make it non-biodegradable, meaning that we must recycle PET to avoid polluting the environment with it. Traditional methods, such as melting and reforming the plastic, destroy the mechanical properties of PET, eliminating the ability to recycle it and necessitating a new strategy for recycling PET[1].
Enzyme hydrolysis
One solution that researchers have been studying is using a naturally occurring enzyme that has PET degrading activity to break down the polymer into its monomers[2]. This would allow the PET to be reformed from its monomers into a new piece of plastic.
A class of enzymes that has been a focus for this goal are Cutinase enzymes.
There are many different pathways to breakdown PET and they are pictured in Figure 2, each posing unique strategies and ways of breaking it down[3]. One simple way to break PET down is through the hydrolysis of the ester bond between each monomer. There are already known enzymes that can perform hydrolysis on ester bonds of polymers, so applying one of these enzymes to PET provides a good starting point for the engineering of a PET hydrolase enzyme, like [4].
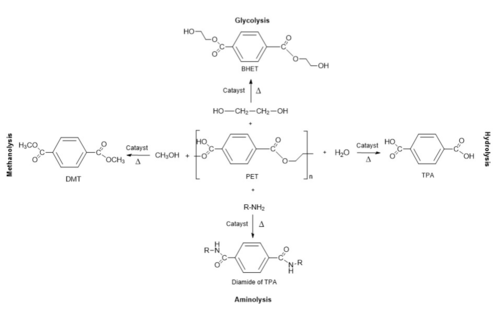
Figure 2 - The different possible pathways of breakdown PET
[3], with our focus being on hydrolysis
Leaf-branch compost cutinase (LCC)
The study done by Tournier et al. (2020)[4] identified a good template enzyme for creating a PET hydrolase as the LCC enzyme. This enzyme was discovered through metagenomics of a leaf-branch compost. Its target substrate is the polymer cutin, found in the cuticle of plants. LCC breaks down Cutin to separate the monomers at the ester bond between them[5]. The LCC enzyme was compared to several other enzymes and was selected as it showed the best rate of PET depolymerization[6].
3-D structure
LCC has a tertiary structure. It contains an α-/β-hydrolase fold, which has a core of [7].
Active Site
LCC is a serine hydrolase and it functions through a : S165, D210, and H242. These key residues along with the , M166 and Y95, and the model substrate being [8] make up the [4] of LCC.
Mechanism
The chemistry involved is covalent catalysis and hydrolysis. D210 and H242 pull electron density away from S165, making it a good nucleophile and allowing it to attack the electrophilic carbonyl carbon with the oxyanion hole stabilizing the two tetrahedral transition states[9] (Figure 3). H242 and D210 also play critical roles in general acid-base catalysis which facilitate leaving groups, step 4, and in helping water attack the electrophilic carbonyl carbon to break the covalent bond between the substrate and S165. Which as result, produces two monomers and resets the enzyme (Figure 3).
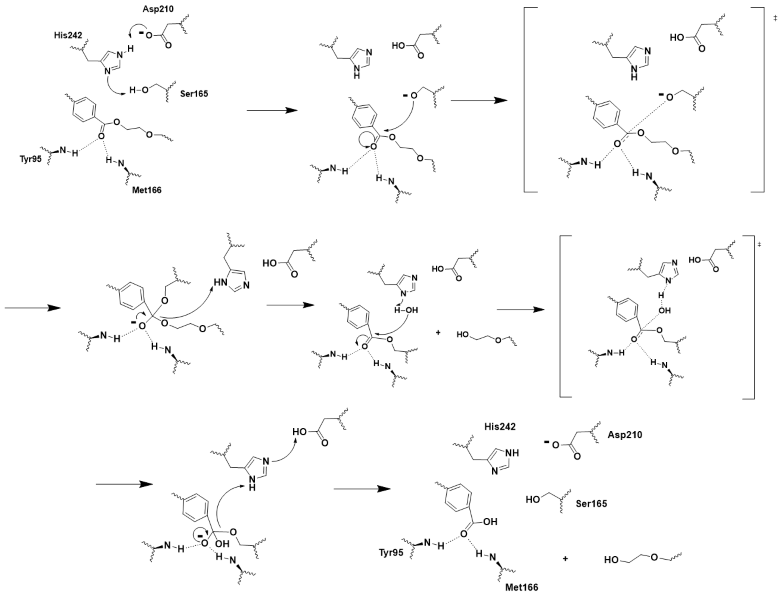
Figure 3 - Push-mechanism for the cutinase reaction
Mutations
After selection, LCC was mutated in order to improve its efficiency and thermal stability. Tournier et al. (2020)[4] identified from the wildtype as being apart of the . They then made hundreds of different mutation commutations, marking which altered efficiency and stability the most. Two of these 11 were chosen for the final mutant enzyme, . These mutations were paired with two separately identified thermostability specific mutations (D238C and S283C) to make the final enzyme.
Efficiency improvement
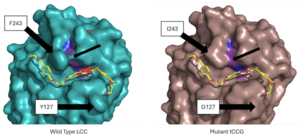
Figure 4 - Protein surface images displaying the impact on the active site
Two of the mutations were selected as they showed the best improvement in catalytic efficiency. The mutation of residue 127 from a to a and the mutation of residue 243 from a to an improved the specific activity of the PET depolymerization of the enzyme by around 27%[4]. Molecular simulations showed that the distance between the carbonyl carbon of the substrate, oxygen of S165, and the nitrogen of H242 was decreased[4]. This increase in catalytic activity was likely due to the decreased average distance between the catalytic residues in the mutant enzyme. This change can be seen in the surface view of the enzyme’s active site seen in Figure 4, which shows the loss of the “bridge” over the active site created by the interaction between F243 and its nearby residues. The loss of these interactions when changed to I243 most likely allows for the relaxation of the active site residues and the decrease in the distance between them. Molecular simulations showed that the distance between the carbonyl carbon of the substrate, oxygen of S165, and the nitrogen of H242 was decreased[4].
Thermostability improvement
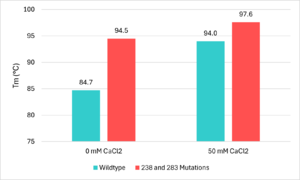
Figure 5 - Thermostability lab data from Tournier et al. (2020)
[4]To allow the enzyme to be effective in the recycling of PET, it needed to be stable in the high temperature environments used during the recycling process. Because of this, improvements needed to be made to the thermostability of the enzyme. The researchers went about this by searching for divalent metal binding sites, which had been seen to improve the thermal stability of other PET hydrolase enzymes[4]. They found this site formed by the residues of . These residues were picked from LCC due to being in the same location of a metal binding site that is found on . All 3 including LCC shared the same 2 to 3 acidic residues which allow a calcium or magnesium ion to bind. that had been seen in other PET hydrolase enzymes, and when calcium ions were added to the enzyme the thermal stability improved. In order to avoid issues with purification, the team opted to avoid the addition of calcium salts and to instead create a disulfide bridge at the location of the divalent metal binding site. They chose to replace the with cysteine residues to form this . This mutation improved the melting temperature of the enzyme by 9.8℃[4]. Figure 5 uses this thermostability data and displays visually the effects the mutations have on melting temperatures[4].
Conclusions
The mutagenesis of LCC into the ICCG PET hydrolase enzyme has proved to be a major success. The specifically chosen mutations (Y127G, F243I, D238C, S283C) led to both a 27% improvement in enzyme efficiency and a nearly 10℃ improvement in the melting point of the enzyme. These improvements prove that the ability to create a commercially viable PET hydrolase recycling process is possible.
Future directions
The development of the ICCG PET Hydrolase enzyme still has room for improvement. To start, future mutagenesis can be conducted to see if the efficiency of the enzyme can be improved further. Some possible modifications could be A59K, V63I, or N248P.[2] ICCG isn’t the only enzyme that is available for modification either, other known PET Hydrolase enzymes, such as IsPETase, could be modified by a similar mutagenesis process.[2] Other degradation processes could be examined to determine their effectiveness in degrading PET. For example, glycolysis could be the next type of degradation to explore. It involves the breakdown of PET in the presence of glycols using metal-oxide based catalysts and can be performed at lower temperatures than hydrolysis.[4] Finally, it could also be possible to use this mutagenesis process to improve the ability of other hydrolases to break down different classes of plastics.[2].





