This is a default text for your page Vinícius M. Neto/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Basic structure
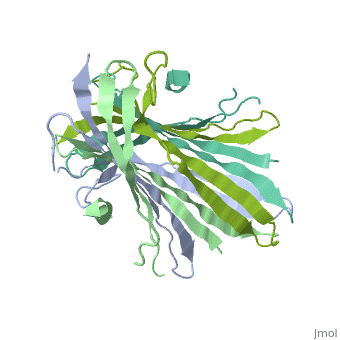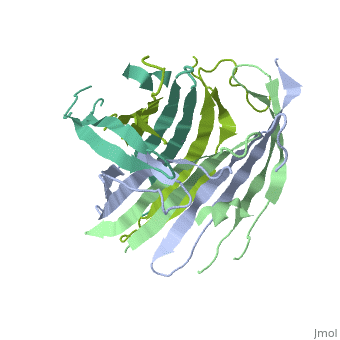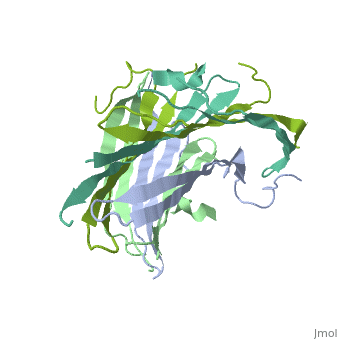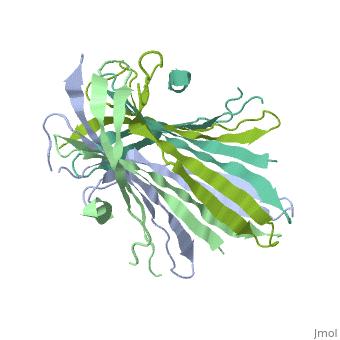
The N-Terminal domain of the fibroin heavy chain (fibNT 3UA0) is a homo 4-mer composed of 268 residues, being most of them (hidrophilic amino acids in marron and hidrophobic in medium blue). FibNTs is a homodimer with 8 alternated β-sheets and a disordered C-terminus portion (Gly109-Ser126). Its two chains (chain A and chain B) are roughly the same but for the N-terminal segments (Phe26-Val35), that form a short helix packing in the chain A and a loop in the B.
Oligomerization
At a neutral pH, the FibNT exists in a random coil state, which prevents premature formation of β-sheets. As the pH decreases to around 6.0, FibNT undergoes a structural transition to form β-sheets. This pH drop mirrors the natural process occurring as fibroin moves from the posterior to the anterior silk gland of the silkworm.FibNT undergoes a conformational change from a random coil to β-sheets when the pH decreases to around 6.0. This transition is critical for dimerization and subsequent oligomerization.
Dynamic light scattering (DLS) and electron microscopy (EM) show that FibNT forms micelle-like oligomers as pH decreases. This suggests that FibNT acts as a pH-sensitive module, initiating self-assembly under acidic conditions similar to those in the silk gland lumen.
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.