User:Laura Fountain/Chloride Ion Channel
From Proteopedia
Contents |
CLIC1: A Chloride Ion Channel
The CLIC family consists of seven members: CLIC1-5, p64, and parchorin. CLIC1 is the most commonly studied member of the CLIC family because it is expressed to some extent in most tissues and cell types that have been studied and is particularly highly expressed in muscle.[1] Along with being present in the plasma membrane, CLIC1 has been found in various intracellular membranes, such as those of the mitochondria, nucleus (where it is designated NCC27), vesicles, and the endoplasmic reticulum.[2][3]
This wide range of locations in the cell causes a plausible reason to assume that the CLIC chloride channel family participate in an equally wide variety of physiological processes. Some of these include cell division, kidney function, bone resorption, transepithelial transport, and signal transduction. [2]
CLIC1 is a member of the highly conserved class of chloride ion channels that exist in both soluble and integral membrane forms. When disrupted cells are washed approximately half of the CLIC1 proteins will remain within the fractioned membrane as would be expected from an integral membrane protein. Atypically, the other half will behave as a soluble cytoplasmic protein and exist within the aqueous extract.[1]
This is part of the evidence which leads Tulk et. al. to postulate that CLIC1 is among the small group of proteins which are assembled as soluble cytoplasmic proteins, which will then insert themselves into the appropriate membrane via their own mechanism.
Structure
|
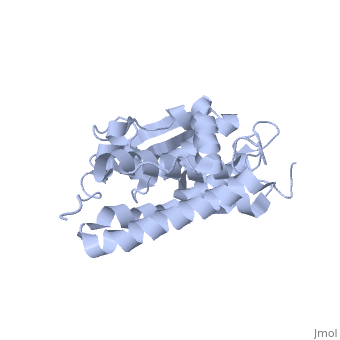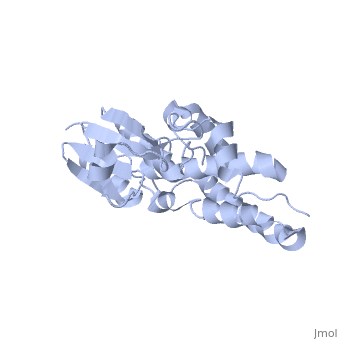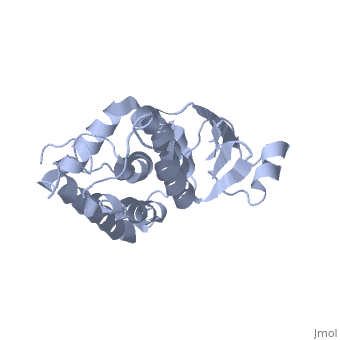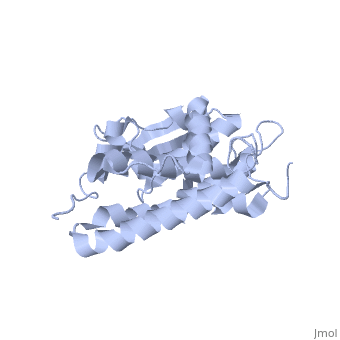
The CLIC family is defined by a COOH-terminal core segment of ~230 amino acids that are highly conserved among the family members. CLIC1 only contains a few amino acids upstream of the conserved core.[1] PUT IN SCENE WITH CONSERVATION COLORS
The N-domain of CLIC1 (1-90) consists of 4 beta-sheets and 3 alpha-helices and the C-domain consists entirely of alpha-helices. The long loop between helices h5 and h6 at the foot of CLIC1 (Pro147–Gln164) is a distinctive feature of the CLICs. It is highly negatively charged with seven acidic residues between Pro149 and Glu160[3] (from Cys24 to Val46 in CLIC1 is Tulk's proposed TM helix. The central 10 amino acids (Leu30–Val39) are nonpolar (except Lys37), while the flanking six to seven residues are more polar in character with two conserved phenylalanines (Phe26 and Phe41). This pattern is typical for transmembrane helicescortex vesicles (50)). Given the proximity of the slot to the GSH binding site, the mechanisms of GSH and IAA-94 in CLIC1 are likely to be related.
The structure of the soluble form of CLIC1 has been determined at 1.4-A resolution, and is shown to the right.[3] It has a homodimeric structure with one pore per subunit, which creates an incredibly unique "double barreled" channel. Integration of CLIC1 into the membrane is likely to require a major structural rearrangement, probably of the N-domain (), with the putative transmembrane helix arising from residues in the vicinity of the redox-active site.[3]
While this exact mechanism isn't known, it has been shown that functionality of the channel doesn't change whether it goes through 'normal' membrane integration via vesicles, or whether it's inserted into the intracellular space and allowed to integrate itself.[1]
Selectivity for Chloride
Based on the similarity of CLIC1's antiparallel alpha helical loop (101-145) to those of other better understood proteins which are able to insert themselves into the membrane, Tulk et. al. propose that this is the single area of the protein which is able to transverse the membrane and also serve as a pore. The 5 positively and 5 negatively charged amino acids on each end of the alpha helices could be part of the ion selectivity of this channel. [1]
Potential Ion Gating
At its binding site in the pore, chloride interacts with the ends of four helices that come from both sides of the membrane. A that protrudes into the pore is proposed to participate in gating.[4]
Function Within Cell
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002 May;282(5):C1103-12. PMID:11940526 doi:10.1152/ajpcell.00402.2001
- ↑ 2.0 2.1 Cromer BA, Morton CJ, Board PG, Parker MW. From glutathione transferase to pore in a CLIC. Eur Biophys J. 2002 Sep;31(5):356-64. Epub 2002 May 23. PMID:12202911 doi:10.1007/s00249-002-0219-1
- ↑ 3.0 3.1 3.2 3.3 Harrop SJ, DeMaere MZ, Fairlie WD, Reztsova T, Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Jankova L, Warton K, Bauskin AR, Wu WM, Pankhurst S, Campbell TJ, Breit SN, Curmi PM. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolution. J Biol Chem. 2001 Nov 30;276(48):44993-5000. Epub 2001 Sep 10. PMID:11551966 doi:10.1074/jbc.M107804200
- ↑ Estevez R, Jentsch TJ. CLC chloride channels: correlating structure with function. Curr Opin Struct Biol. 2002 Aug;12(4):531-9. PMID:12163078