Sandbox 1b41
From Proteopedia
Human Acetylcholinesterase (1b41)
| |||||||||
| 1b41, resolution 2.76Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | ACHE (Homo sapiens) | ||||||||
| Activity: | Acetylcholinesterase, with EC number 3.1.1.7 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
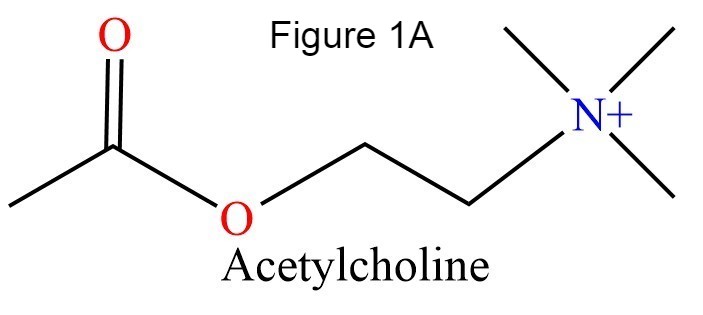
The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter Acethylcholin (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. It has an ellipsoidal shape with dimensions ~ 4,5nm x 6nm x 6,5nm. It consists of 12-stranded, central mixed β-sheet surrounded by 14 α helices.
In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). The AChE is linked with these anchoring molecules by a "tryptophan amphiphilic tetramerization" domain (WAT). There is also a monomeric form which is soluble in the blood.
The Active site gorge of AChE
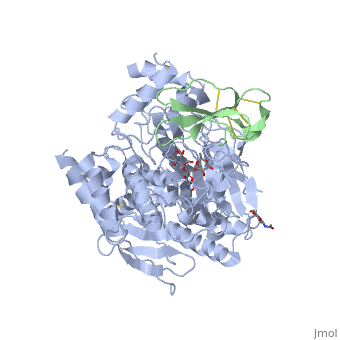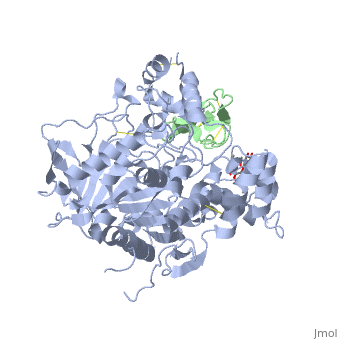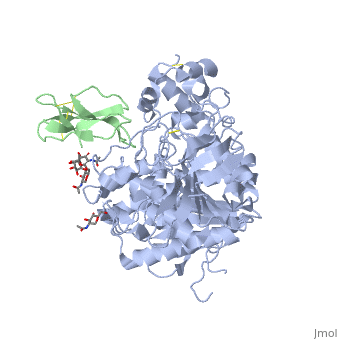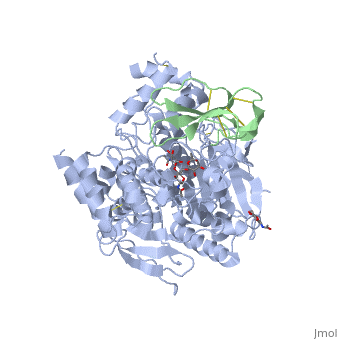
The active site of AChE involved two sites: the peripheral site and the catalytic site (Figure ).
The peripheral site is a transitional binding site of the substrate. It provides a region rich in aromatic amino acids that guide the ligands (ACh or other agonists) by setting an array of low-affinity binding sites. This hydrophobic region trapps ACh and transfers it to the deep catalytic site. (PyMol)
The catalytic site of AChE consists of two subsites: the "esteratic" site and "the anionic" site. (figure)
In the "esteratic" site a catalytic triad consisting of E334, H447, S203 forms a planar array that ressembles the catalytic triad of serine proteases. S203 is activated (it becomes nucleophilic) by E334 and H447. This activation allows the following reaction: the acylation between hydroxyl group of S203 and ACh oxygen (or other agonists). A covalent bond between the enzyme and the substrate creates an oxyanion. This oxyanion then reacts with two glycins setting up hydrogen bond. In the "anionic" site, the W86 binds trimethylammonium group of ACh.
Further to these steps the substrat is well positioned to be hydrolysed.
Inhibitors of AChE
Fasciculin II Fasciculin is a snake toxin. It's a little protein of 7kDa which inhibits AChE in bindind the peripheric site, preventing the substrate from passage through the narrower portion of the gorge towards the catalytic site. This inhibition is almost irreversible. The toxin is the one used in cristallisation of the Human acetylcholinesterase (in green on the picture).
Inhibitors used as treatments We can find a lot of inhibitors such as Alzeihmer's disease drugs treatment. These treatment include rivastigmine, donepezil and tacrine.